-
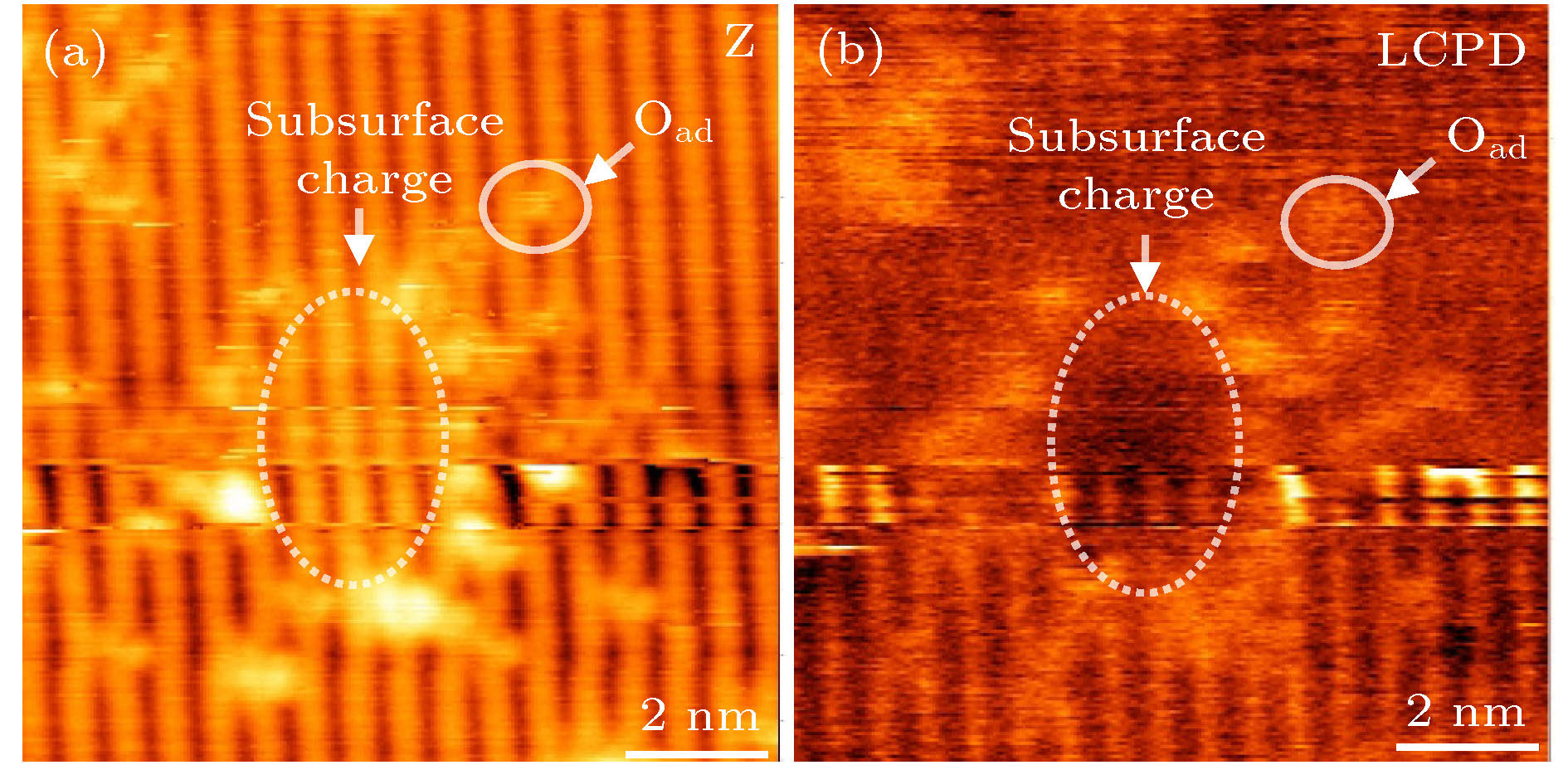
半导体金属氧化物二氧化钛因其稳定、高效、无毒、兼容、低廉等优点受到广泛的关注, 本文在低温78 K下利用原子力显微镜探索了二氧化钛亚表面电荷的特性及其对表面点缺陷和吸附原子分布的影响. 在原子结构形貌图中亚表面电荷被成像为椭圆形亮丘, 且亮丘的高度主要分布在3个不同的区间内, 这意味着亚表面电荷位于3个不同的亚表面原子层. 在开尔文探针力显微镜成像中, 亚表面电荷的电势分布相对低, 根据电势成像机理, 亚表面电荷为正电荷特性. 原子力显微镜的成像图表明, 亚表面电荷不仅排斥具有正电荷特性的表面氧空位、吸附氢原子和表面台阶, 而且排斥具有负电荷特性的吸附氧原子. 实验结果有助于二氧化钛物理特性的研究及其相关产品性能的改进和设计.
Transition-metal-oxide as a typical model surface for investigating the catalytic mechanism has been widely studied. Over the past years, the TiO2 properties have been reported. It is commonly accepted that the catalytic activity of reduced TiO2 is related to its defects, with the accompanying excess electrons leading to n-type conductivity. It is realized that subsurface charge is of key importance for the redox chemistry of TiO2 (110). Subsurface charge is explored by atomic force microscopy (AFM) and Kelvin probe force microscopy (KPFM). Subsurface charge exerts an additional attractive force on the scanning AFM tip, resulting in the relative retraction of tip motion in order to keep a constant frequency shift. As a result, the subsurface charged region is imaged as protrusion in an AFM topographic image. The height of bright hillock is mainly distributed in three different ranges, which means that the subsurface charges are at three different subsurface layers. The AFM results show such subsurface charges repel the electropositive oxygen vacancy, hydrogen atoms and step edges. It is obvious that there is not only an Ov depletion zone but also the subsurface charge free region in the proximity of the $\left\langle {001} \right\rangle $ and$\left\langle {1\bar 11} \right\rangle$ step edge.The KPFM image indicates that the subsurface charges are the positive charges. which is consistent with common sense. After oxygen exposure, it is found that the oxygen adatom is electronegative, but it is absent in the vicinity of positive subsurface charges. Irrespective of adsorbate being electropositive or electronegative, an adsorbate-free zone generally exists in the proximity of the charged region. Obviously, the present study is expected to provide some insights into clarifying the nature of subsurface charge and improving catalytic design. -
Keywords:
- TiO2 /
- subsurface charge /
- atomic force microscopy /
- Kelvin probe force microscopy
[1] Fujishima A, Honda K 1972 Nature 238 37
 Google Scholar
Google Scholar
[2] Diebold U 2003 Surf. Sci. Rep. 48 53
 Google Scholar
Google Scholar
[3] Pang C L, Lindsay R, Thornton G 2008 Chem. Soc. Rev. 37 2328
 Google Scholar
Google Scholar
[4] Henderson M A, Lyubinetsky I 2013 Chem. Rev. 113 4428
 Google Scholar
Google Scholar
[5] Wen H F, Adachi Y, Zhang Q, Miyazaki M, Sugawara Y, Li Y J 2019 J. Phys. Chem. C 123 25756
 Google Scholar
Google Scholar
[6] Guo Q, Ma Z, Zhou C, Ren Z, Yang X 2019 Chem. Rev. 119 11020
 Google Scholar
Google Scholar
[7] Guo C, Meng X, Fu H, Wang Q, Wang H, Tian Y, Peng J, Ma R, Weng Y, Meng S, Wang E, Jiang Y 2020 Phys. Rev. Lett. 124 206801
 Google Scholar
Google Scholar
[8] Tan S, Feng H, Zheng Q, Cui X, Zhao J, Luo Y, Yang J, Wang B, Hou J G 2020 J. Am. Chem. Soc. 142 826
 Google Scholar
Google Scholar
[9] Wen H F, Miyazaki M, Zhang Q, Adachi Y, Li Y J, Sugawara Y 2018 Phys. Chem. Chem. Phys. 20 28331
 Google Scholar
Google Scholar
[10] Batzill M, Katsiev K, Gaspar D J, Diebold U 2002 Phys. Rev. B 66 235401
 Google Scholar
Google Scholar
[11] Wendt S, Schaub R, Sprunger P T, Lira E, Madsen G K H, Li Z, Hansen J O, Matthiesen J, Blekinge-Rasmussen A, Lægsgaard E, Hammer B, Besenbacher F 2008 Science 320 1755
 Google Scholar
Google Scholar
[12] 李天晶, 李公平, 马俊平, 高行新 2011 60 116102
 Google Scholar
Google Scholar
Li T J, Li G P, Ma J P, Gao X X 2011 Acta Phys. Sin. 60 116102
 Google Scholar
Google Scholar
[13] 薛将, 潘风明, 裴煜 2013 62 158103
 Google Scholar
Google Scholar
Xu J, Pan F M, Pei Y 2013 Acta Phys. Sin. 62 158103
 Google Scholar
Google Scholar
[14] 朱学文, 徐利春, 刘瑞萍, 杨致, 李秀燕 2015 64 147103
 Google Scholar
Google Scholar
Zhu X M, Xu L C, Liu R P, Yang Z, Li X Y 2015 Acta Phys. Sin. 64 147103
 Google Scholar
Google Scholar
[15] 朱伟君, 陈金鑫, 高宇晗, 杨德仁, 马向阳 2019 68 124204
 Google Scholar
Google Scholar
Zhu W J, Chen J X, Gao Y H, Yang D R, Ma X Y 2019 Acta Phys. Sin. 68 124204
 Google Scholar
Google Scholar
[16] Yoon Y, Du Y, Garcia J C, Zhu Z, Wang Z T, Petrik N G, Kimmel G A, Dohnalek Z, Henderson M A, Rousseau R, Deskins N A, Lyubinetsky I 2015 Chem. Phys. Chem. 16 313
 Google Scholar
Google Scholar
[17] Feng H, Xu Z, Ren L, Liu C, Zhuang J, Hu Z, Xu X, Chen J, Wang J, Hao W, Du Y, Dou S X 2018 ACS Catal. 8 4288
 Google Scholar
Google Scholar
[18] Binnig G, Quate C. F, Gerber C. H 1986 Phys. Rev. Lett. 56 930
 Google Scholar
Google Scholar
[19] García R, Pérez R 2002 Surf. Sci. Rep. 47 197
 Google Scholar
Google Scholar
[20] Jiang P, Bao X, Salmeron M 2015 Acc. Chem. Res. 48 1524
 Google Scholar
Google Scholar
[21] Zhang J, Chen P C, Yuan B K, Ji W, Cheng Z H, Qiu X H 2013 Science 342 611
 Google Scholar
Google Scholar
[22] Guo J, Meng X Z, Chen J, Peng J B, Sheng J M, Li X Z, Xu L M, Shi J R, Wang E G, Jiang Y 2014 Nat. Mater. 13 184
 Google Scholar
Google Scholar
[23] Ma R, Cao D, Zhu C, Tian Y, Peng J, Guo J, Chen J, Li X Z, Francisco J S, Zeng X C, Xu L M, Wang E G, Jiang Y 2020 Nature 577 60
 Google Scholar
Google Scholar
[24] Li Y J, Wen H F, Zhang Q, Adachi Y, Arima E, Kinoshita Y, Nomura H, Ma Z, Kou L, Tsukuda Y, Naitoh Y, Sugawara Y, Xu R, Cheng Z H 2018 Ultramicroscopy 191 51
 Google Scholar
Google Scholar
[25] Kinoshita Y, Naitoh Y, Li Y J, Sugawara Y 2011 Rev. Sci. Instrum. 82 113707
 Google Scholar
Google Scholar
[26] Zhang Z, Ge Q, Li S C, Kay B D, White J M, Dohnalek Z 2007 Phys. Rev. Lett. 99 126105
 Google Scholar
Google Scholar
[27] Lauritsen J V, Foster A S, Olesen G H, Christensen M C, Kühnle A, Helveg S, Rostrup-Niesen J R, Clausen B S, Reichling M, Besenbacher F 2006 Nanotechnology 17 3436
 Google Scholar
Google Scholar
[28] Bechstein R, González C, Schütte J, Jelínek P, Pérez R, Kühnle A 2009 Nanotechnology 20 505703
 Google Scholar
Google Scholar
[29] Yurtsever A, Sugimoto Y, Abe M, Morita S 2010 Nanotechnology 21 165702
 Google Scholar
Google Scholar
[30] Ishida N, Fujita D J 2012 J. Phys. Chem. C 116 20300
 Google Scholar
Google Scholar
[31] Byl O, Yates J T Jr 2006 J. Phys. Chem. B 110 22966
 Google Scholar
Google Scholar
[32] Smoluchowski, R 1941 Phys. Rev. 60 661
 Google Scholar
Google Scholar
[33] Miyazaki M, Wen H F, Zhang Q, Adachi Y, Brndiar J, Štich I, Li Y J, Sugawara Y 2019 Beilstein J. Nanotechnol. 10 1228
 Google Scholar
Google Scholar
[34] Zhang Q, Li Y J, Wen H F, Adachi Y, Miyazaki M, Sugawara Y, Xu R, Cheng Z H, Brndiar J, Kantorovich L, Štich I 2018 J. Am. Chem. Soc. 140 15668
 Google Scholar
Google Scholar
-
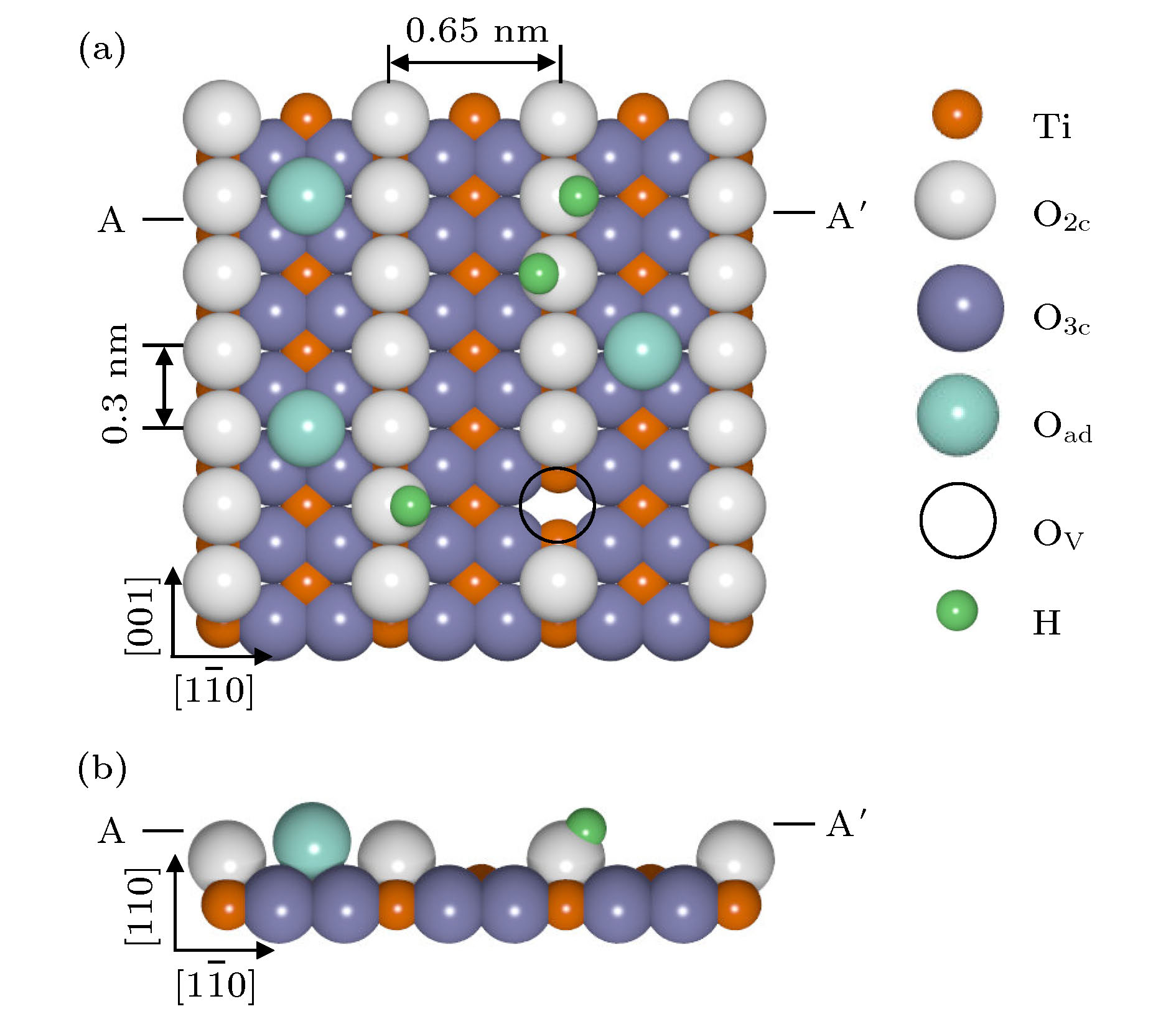
图 1 金红石TiO2 (110)-(1 × 1)的表面模型结构的 (a)俯视图和(b)侧视图(由交替的五配位Ti5c列(橙色球)和六配位的Ti6c组成; Ti6c列被共面的三配位氧气O3c列(蓝色球)和二配位桥氧O2c列(灰色球)所包围; 表面缺陷主要包含Ov (虚黑圈)和OH缺陷(绿色小球))
Fig. 1. (a) Top view and (b) side view of ball model of rutile TiO2 (110)-(1 × 1) surface consist of alternating O2c (gray ball) and Ti5c (orange ball) atom rows. H atom (green small ball) is generated by water dissociation on Ov site, and O adatom (light green large ball) on Ti5c row is formed by O2 dissociation on Ov and/or Ti5c sites.
图 2 TiO2 (110)样品表面的代表性AFM形貌图 (a)和(b)中亮列是O2c, 暗点是表面缺陷; (c)中亮列是Ti5c, 亮点是缺陷; (d)是亮丘的轮廓线, 表明亚表面缺陷分布于3个不同的亚表面原子层.
Fig. 2. AFM topographic images of TiO2 (110) surface obtained at different experiments at 78 K. (a), (b) The bright rows are the O2c row, and dark spots are the surface defects. (c) The bright rows are the Ti5c row. Bright spot is the Ov, and brighter spots are the H atoms. Bright regions are observed in all three images. (d) Line profiles cross the bright region along the
$ \left[ {1\bar10} \right] $ direction on TiO2 (110) surface, taken from different AFM images. The height shows three specific regions, corresponding to three different depths of subsurface charge. -
[1] Fujishima A, Honda K 1972 Nature 238 37
 Google Scholar
Google Scholar
[2] Diebold U 2003 Surf. Sci. Rep. 48 53
 Google Scholar
Google Scholar
[3] Pang C L, Lindsay R, Thornton G 2008 Chem. Soc. Rev. 37 2328
 Google Scholar
Google Scholar
[4] Henderson M A, Lyubinetsky I 2013 Chem. Rev. 113 4428
 Google Scholar
Google Scholar
[5] Wen H F, Adachi Y, Zhang Q, Miyazaki M, Sugawara Y, Li Y J 2019 J. Phys. Chem. C 123 25756
 Google Scholar
Google Scholar
[6] Guo Q, Ma Z, Zhou C, Ren Z, Yang X 2019 Chem. Rev. 119 11020
 Google Scholar
Google Scholar
[7] Guo C, Meng X, Fu H, Wang Q, Wang H, Tian Y, Peng J, Ma R, Weng Y, Meng S, Wang E, Jiang Y 2020 Phys. Rev. Lett. 124 206801
 Google Scholar
Google Scholar
[8] Tan S, Feng H, Zheng Q, Cui X, Zhao J, Luo Y, Yang J, Wang B, Hou J G 2020 J. Am. Chem. Soc. 142 826
 Google Scholar
Google Scholar
[9] Wen H F, Miyazaki M, Zhang Q, Adachi Y, Li Y J, Sugawara Y 2018 Phys. Chem. Chem. Phys. 20 28331
 Google Scholar
Google Scholar
[10] Batzill M, Katsiev K, Gaspar D J, Diebold U 2002 Phys. Rev. B 66 235401
 Google Scholar
Google Scholar
[11] Wendt S, Schaub R, Sprunger P T, Lira E, Madsen G K H, Li Z, Hansen J O, Matthiesen J, Blekinge-Rasmussen A, Lægsgaard E, Hammer B, Besenbacher F 2008 Science 320 1755
 Google Scholar
Google Scholar
[12] 李天晶, 李公平, 马俊平, 高行新 2011 60 116102
 Google Scholar
Google Scholar
Li T J, Li G P, Ma J P, Gao X X 2011 Acta Phys. Sin. 60 116102
 Google Scholar
Google Scholar
[13] 薛将, 潘风明, 裴煜 2013 62 158103
 Google Scholar
Google Scholar
Xu J, Pan F M, Pei Y 2013 Acta Phys. Sin. 62 158103
 Google Scholar
Google Scholar
[14] 朱学文, 徐利春, 刘瑞萍, 杨致, 李秀燕 2015 64 147103
 Google Scholar
Google Scholar
Zhu X M, Xu L C, Liu R P, Yang Z, Li X Y 2015 Acta Phys. Sin. 64 147103
 Google Scholar
Google Scholar
[15] 朱伟君, 陈金鑫, 高宇晗, 杨德仁, 马向阳 2019 68 124204
 Google Scholar
Google Scholar
Zhu W J, Chen J X, Gao Y H, Yang D R, Ma X Y 2019 Acta Phys. Sin. 68 124204
 Google Scholar
Google Scholar
[16] Yoon Y, Du Y, Garcia J C, Zhu Z, Wang Z T, Petrik N G, Kimmel G A, Dohnalek Z, Henderson M A, Rousseau R, Deskins N A, Lyubinetsky I 2015 Chem. Phys. Chem. 16 313
 Google Scholar
Google Scholar
[17] Feng H, Xu Z, Ren L, Liu C, Zhuang J, Hu Z, Xu X, Chen J, Wang J, Hao W, Du Y, Dou S X 2018 ACS Catal. 8 4288
 Google Scholar
Google Scholar
[18] Binnig G, Quate C. F, Gerber C. H 1986 Phys. Rev. Lett. 56 930
 Google Scholar
Google Scholar
[19] García R, Pérez R 2002 Surf. Sci. Rep. 47 197
 Google Scholar
Google Scholar
[20] Jiang P, Bao X, Salmeron M 2015 Acc. Chem. Res. 48 1524
 Google Scholar
Google Scholar
[21] Zhang J, Chen P C, Yuan B K, Ji W, Cheng Z H, Qiu X H 2013 Science 342 611
 Google Scholar
Google Scholar
[22] Guo J, Meng X Z, Chen J, Peng J B, Sheng J M, Li X Z, Xu L M, Shi J R, Wang E G, Jiang Y 2014 Nat. Mater. 13 184
 Google Scholar
Google Scholar
[23] Ma R, Cao D, Zhu C, Tian Y, Peng J, Guo J, Chen J, Li X Z, Francisco J S, Zeng X C, Xu L M, Wang E G, Jiang Y 2020 Nature 577 60
 Google Scholar
Google Scholar
[24] Li Y J, Wen H F, Zhang Q, Adachi Y, Arima E, Kinoshita Y, Nomura H, Ma Z, Kou L, Tsukuda Y, Naitoh Y, Sugawara Y, Xu R, Cheng Z H 2018 Ultramicroscopy 191 51
 Google Scholar
Google Scholar
[25] Kinoshita Y, Naitoh Y, Li Y J, Sugawara Y 2011 Rev. Sci. Instrum. 82 113707
 Google Scholar
Google Scholar
[26] Zhang Z, Ge Q, Li S C, Kay B D, White J M, Dohnalek Z 2007 Phys. Rev. Lett. 99 126105
 Google Scholar
Google Scholar
[27] Lauritsen J V, Foster A S, Olesen G H, Christensen M C, Kühnle A, Helveg S, Rostrup-Niesen J R, Clausen B S, Reichling M, Besenbacher F 2006 Nanotechnology 17 3436
 Google Scholar
Google Scholar
[28] Bechstein R, González C, Schütte J, Jelínek P, Pérez R, Kühnle A 2009 Nanotechnology 20 505703
 Google Scholar
Google Scholar
[29] Yurtsever A, Sugimoto Y, Abe M, Morita S 2010 Nanotechnology 21 165702
 Google Scholar
Google Scholar
[30] Ishida N, Fujita D J 2012 J. Phys. Chem. C 116 20300
 Google Scholar
Google Scholar
[31] Byl O, Yates J T Jr 2006 J. Phys. Chem. B 110 22966
 Google Scholar
Google Scholar
[32] Smoluchowski, R 1941 Phys. Rev. 60 661
 Google Scholar
Google Scholar
[33] Miyazaki M, Wen H F, Zhang Q, Adachi Y, Brndiar J, Štich I, Li Y J, Sugawara Y 2019 Beilstein J. Nanotechnol. 10 1228
 Google Scholar
Google Scholar
[34] Zhang Q, Li Y J, Wen H F, Adachi Y, Miyazaki M, Sugawara Y, Xu R, Cheng Z H, Brndiar J, Kantorovich L, Štich I 2018 J. Am. Chem. Soc. 140 15668
 Google Scholar
Google Scholar
计量
- 文章访问数: 10328
- PDF下载量: 195
- 被引次数: 0















 下载:
下载: