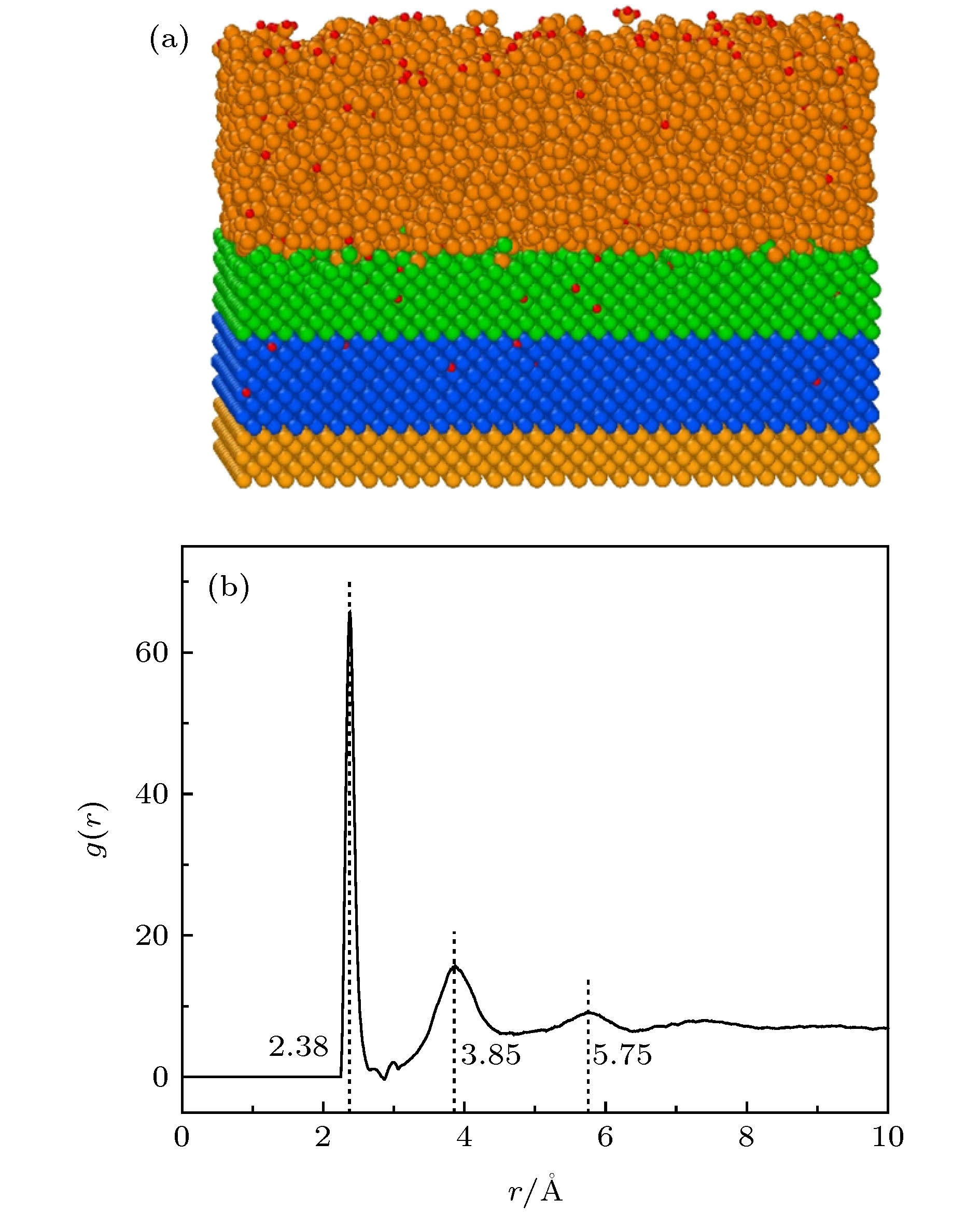
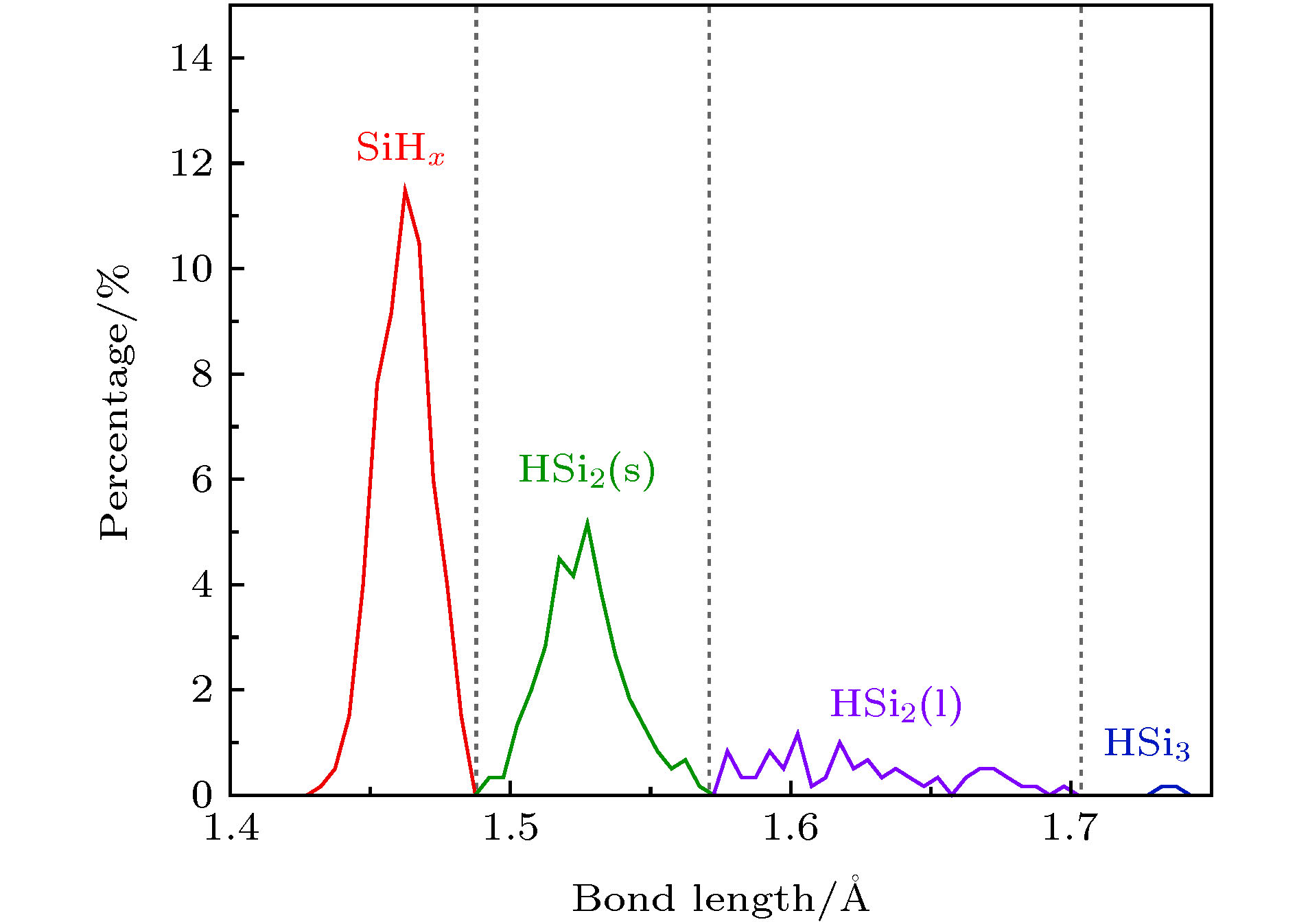
-
氢化非晶硅薄膜(a-Si:H)中SiyHx结构组态对薄膜应用性能有重要影响, 然而现有的分析测试手段难以对其进行深入细致的研究. 本文运用分子动力学方法模拟分析了a-Si:H/c-Si薄膜中SiyHx结构组态, 以及衬底温度对其含量的影响; 并进一步运用第一性原理方法计算了各SiyHx组态中的Si-H键能. 结果发现a-Si:H薄膜中SiyHx结构可以归纳为六种组态. 三类为以化学键结合的SiHx组态, 包括SiH, SiH2和SiH3; 另外三类为以物理键结合的HSiy组态, 包括HSi2(s), HSi2(l)和HSi3. 键能结果反映出六种组态的稳定性由高到低的顺序为SiH > SiH2 > SiH3 > HSi2(s) > HSi2(l) > HSi3. HSiy组态中Si—H键能在太阳光中的可见光和红外线的能量范围内, 阳光照射引起HSiy组态中的Si-H物理键断裂, 是非晶硅薄膜电池产生S-W (Steabler-Wronski)效应的主要机理. 另外, 薄膜沉积过程中衬底温度的升高将导致各类SiyHx组态含量大幅降低.The hydrogenated amorphous silicon (a-Si:H) film is the core structure of hetero junction with intrinsic thin layer solar cell. Its quality determinates the photoelectric conversion efficiency of this solar cell directly. The configuration of SiyHx is an important structure characteristic of a-Si:H films, and it can influence on the quality of a-Si:H thin films and their application properties. However, it is difficult to study them in depth and detail by the existing analytical and testing methods. In this paper, the structure configuration of SiyHx in a-Si:H /c-Si thin films and the effect of substrate temperature on its content have been simulated and analyzed by molecular dynamics method. A modified Tersoff potential developed by Murty was used to calculate the inter-atomic forces. The results showed that the SiyHx structure in a-Si:H thin films can be summarized into six configurations. Three traditional configurations, including SiH, SiH2 and SiH3, can be referred to as SiHx configurations.The other three nove configurations, including HSi2(s), HSi2(l) and HSi3, can be referred to as HSiy configurations. The main differences between the configurations of HSi2(l) and HSi2(s) are the longer Si—H bonds and bigger bond angle in HSi2(l) configuration than those in HSi2(s) configuration. All of the Si-H bonds in SiHx configurations are strong chemical bonds, while the Si—H bonds in HSiyconfigurations are weak physical bonds. The further calculations of the Si-H bond energies in six configurations have been carried out by the first principle method. According the bond energies results, we can deduce that the order of the stability of six configurations from high to low is SiH > SiH2 > SiH3 > HSi2(s) > HSi2(l) > HSi3. Comparing the Si—H bond energies of the six configurations with the solar energy, it is found that the Si-H bond energy in HSiy configuration is in the range of visible and infrared light in solar light. Si—H physical bonds are easy to fracture in HSiy configuration caused by solar light. This may be the main mechanism of producing Steabler-Wronski (S-W) effect in amorphous silicon thin film cells. In addition, the rise of substrate temperature in the deposition process of a-Si:H films will lead to a significant decrease in the configuration content of all kinds of SiyHx configurations.
-
Keywords:
- SiyHx configuration /
- bond energy /
- hydrogenated amorphous silicon thin films (a-Si:H) /
- molecular dynamics
[1] Yoshikawa K, Kawasaki H, Yoshida W, Irie T, Konishi K, Nakano K, Uto T, Adachi D, Kanematsu M, Uzu H, Yamamoto K 2017 Nat. Energy 2 17032
 Google Scholar
Google Scholar
[2] Liu J, Huang S H, He L 2015 J. Semicond. 36 4
 Google Scholar
Google Scholar
[3] Mishima T, Taguchi M, Sakata H, Maruyama E 2011 Sol. Energy Mater. Sol. Cells 95 18
 Google Scholar
Google Scholar
[4] Li Z, Zhang X W, Han G R 2010 Phys. Status Solidi A 207 144
 Google Scholar
Google Scholar
[5] Masuko K, Shigematsu M, Hashiguchi T, Fujishima D, Kai M, Yoshimura N, Yamaguchi T, Ichihashi Y, Mishima T, Matsubara N, Yamanishi T, Takahama T, Taguchi M, Maruyama E, Okamoto S 2014 IEEE. J. Photovoltaics 4 1433
 Google Scholar
Google Scholar
[6] Luo Y R, Gong H Y, Zhou N G, Huang H B, Zhou L 2018 Appl. Phys. A 124 18
 Google Scholar
Google Scholar
[7] Illiberi A, Creatore M, Kessels W M M, de Sanden M 2010 Phys. Status SolidiR 4 206
 Google Scholar
Google Scholar
[8] Bronsveld P C P, Mates T, Fejfar A, Kocka J, Rath J K, Schropp R E I 2010 Phys. Status Solidi A 207 525
 Google Scholar
Google Scholar
[9] Luo Y, Zhou N, Gong H, Huang H, Lang Z 2018 IOP Conf. Ser.: Mater. Sci. Eng. 284 012006
 Google Scholar
Google Scholar
[10] Hou G F, Fan Q H, Liao X B, Chen C Y, Xiang X B, Deng X M 2011 J. Vac. Sci. Technol., A 29 061201
 Google Scholar
Google Scholar
[11] Andujar J L, Bertran E, Canillas A, Roch C, Morenza J L 1991 J. Vac. Sci. Technol., A 9 2216
 Google Scholar
Google Scholar
[12] Staebler D L, Wronski C R 1977 Appl. Phys. Lett. 31 292
 Google Scholar
Google Scholar
[13] Zhang D, Tavakoliyaraki A, Wu Y, van Swaaij R, Zeman M 2011 Energy Procedia 8 207
 Google Scholar
Google Scholar
[14] Alnuaimi A, Islam K, Nayfeh A 2013 Sol. Energy 98 236
 Google Scholar
Google Scholar
[15] 秦国刚, 孔光临 1988 半导体学报 01 103
 Google Scholar
Google Scholar
Qin G G, Kong G L 1988 J. Semicond. 01 103
 Google Scholar
Google Scholar
[16] Murty M V R, Atwater H A 1995 Phys. Rev. B 51 4889
 Google Scholar
Google Scholar
[17] Tersoff J 1988 Phys. Rev. B 37 6991
 Google Scholar
Google Scholar
[18] Macrae C F, Bruno I J, Chisholm J A, Edgington P R, Wood P A 2008 J. Appl. Crystallogr. 41 466
 Google Scholar
Google Scholar
[19] Hafner J, Kresse G 1997 Properties of Complex Inorganic Solids (New York: Plenum Press) pp69–99
[20] Sriraman S, Agarwal S, Aydil E S, Maroudas D 2002 Nature 418 62
 Google Scholar
Google Scholar
[21] Robertson J 2000 J. Non-Cryst. Solids 266 79
 Google Scholar
Google Scholar
[22] Kim H, Horwitz J S, Kushto G, Piqué A, Kafafi Z H, Gilmore C M, Chrisey D B 2000 J. Appl. Phys. 88 6021
 Google Scholar
Google Scholar
-
图 6 不同衬底温度下沉积生长的a-Si:H/c-Si薄膜中的悬挂键、SiHx和HSiy相对含量(a), SiH, SiH2和SiH3的相对含量(b)和HSi2(s), HSi2(l)和HSi3相对含量(c)
Fig. 6. Relative contents of dangling bonds, SiHx and HSiy(a), relative contents of SiH, SiH2 and SiH3 (b), and relative content of HSi2(s), HSi2 (l) and HSi3 (c) in a-Si:H/c-Si films deposited with different substrate temperatures.
-
[1] Yoshikawa K, Kawasaki H, Yoshida W, Irie T, Konishi K, Nakano K, Uto T, Adachi D, Kanematsu M, Uzu H, Yamamoto K 2017 Nat. Energy 2 17032
 Google Scholar
Google Scholar
[2] Liu J, Huang S H, He L 2015 J. Semicond. 36 4
 Google Scholar
Google Scholar
[3] Mishima T, Taguchi M, Sakata H, Maruyama E 2011 Sol. Energy Mater. Sol. Cells 95 18
 Google Scholar
Google Scholar
[4] Li Z, Zhang X W, Han G R 2010 Phys. Status Solidi A 207 144
 Google Scholar
Google Scholar
[5] Masuko K, Shigematsu M, Hashiguchi T, Fujishima D, Kai M, Yoshimura N, Yamaguchi T, Ichihashi Y, Mishima T, Matsubara N, Yamanishi T, Takahama T, Taguchi M, Maruyama E, Okamoto S 2014 IEEE. J. Photovoltaics 4 1433
 Google Scholar
Google Scholar
[6] Luo Y R, Gong H Y, Zhou N G, Huang H B, Zhou L 2018 Appl. Phys. A 124 18
 Google Scholar
Google Scholar
[7] Illiberi A, Creatore M, Kessels W M M, de Sanden M 2010 Phys. Status SolidiR 4 206
 Google Scholar
Google Scholar
[8] Bronsveld P C P, Mates T, Fejfar A, Kocka J, Rath J K, Schropp R E I 2010 Phys. Status Solidi A 207 525
 Google Scholar
Google Scholar
[9] Luo Y, Zhou N, Gong H, Huang H, Lang Z 2018 IOP Conf. Ser.: Mater. Sci. Eng. 284 012006
 Google Scholar
Google Scholar
[10] Hou G F, Fan Q H, Liao X B, Chen C Y, Xiang X B, Deng X M 2011 J. Vac. Sci. Technol., A 29 061201
 Google Scholar
Google Scholar
[11] Andujar J L, Bertran E, Canillas A, Roch C, Morenza J L 1991 J. Vac. Sci. Technol., A 9 2216
 Google Scholar
Google Scholar
[12] Staebler D L, Wronski C R 1977 Appl. Phys. Lett. 31 292
 Google Scholar
Google Scholar
[13] Zhang D, Tavakoliyaraki A, Wu Y, van Swaaij R, Zeman M 2011 Energy Procedia 8 207
 Google Scholar
Google Scholar
[14] Alnuaimi A, Islam K, Nayfeh A 2013 Sol. Energy 98 236
 Google Scholar
Google Scholar
[15] 秦国刚, 孔光临 1988 半导体学报 01 103
 Google Scholar
Google Scholar
Qin G G, Kong G L 1988 J. Semicond. 01 103
 Google Scholar
Google Scholar
[16] Murty M V R, Atwater H A 1995 Phys. Rev. B 51 4889
 Google Scholar
Google Scholar
[17] Tersoff J 1988 Phys. Rev. B 37 6991
 Google Scholar
Google Scholar
[18] Macrae C F, Bruno I J, Chisholm J A, Edgington P R, Wood P A 2008 J. Appl. Crystallogr. 41 466
 Google Scholar
Google Scholar
[19] Hafner J, Kresse G 1997 Properties of Complex Inorganic Solids (New York: Plenum Press) pp69–99
[20] Sriraman S, Agarwal S, Aydil E S, Maroudas D 2002 Nature 418 62
 Google Scholar
Google Scholar
[21] Robertson J 2000 J. Non-Cryst. Solids 266 79
 Google Scholar
Google Scholar
[22] Kim H, Horwitz J S, Kushto G, Piqué A, Kafafi Z H, Gilmore C M, Chrisey D B 2000 J. Appl. Phys. 88 6021
 Google Scholar
Google Scholar
计量
- 文章访问数: 10734
- PDF下载量: 155
- 被引次数: 0













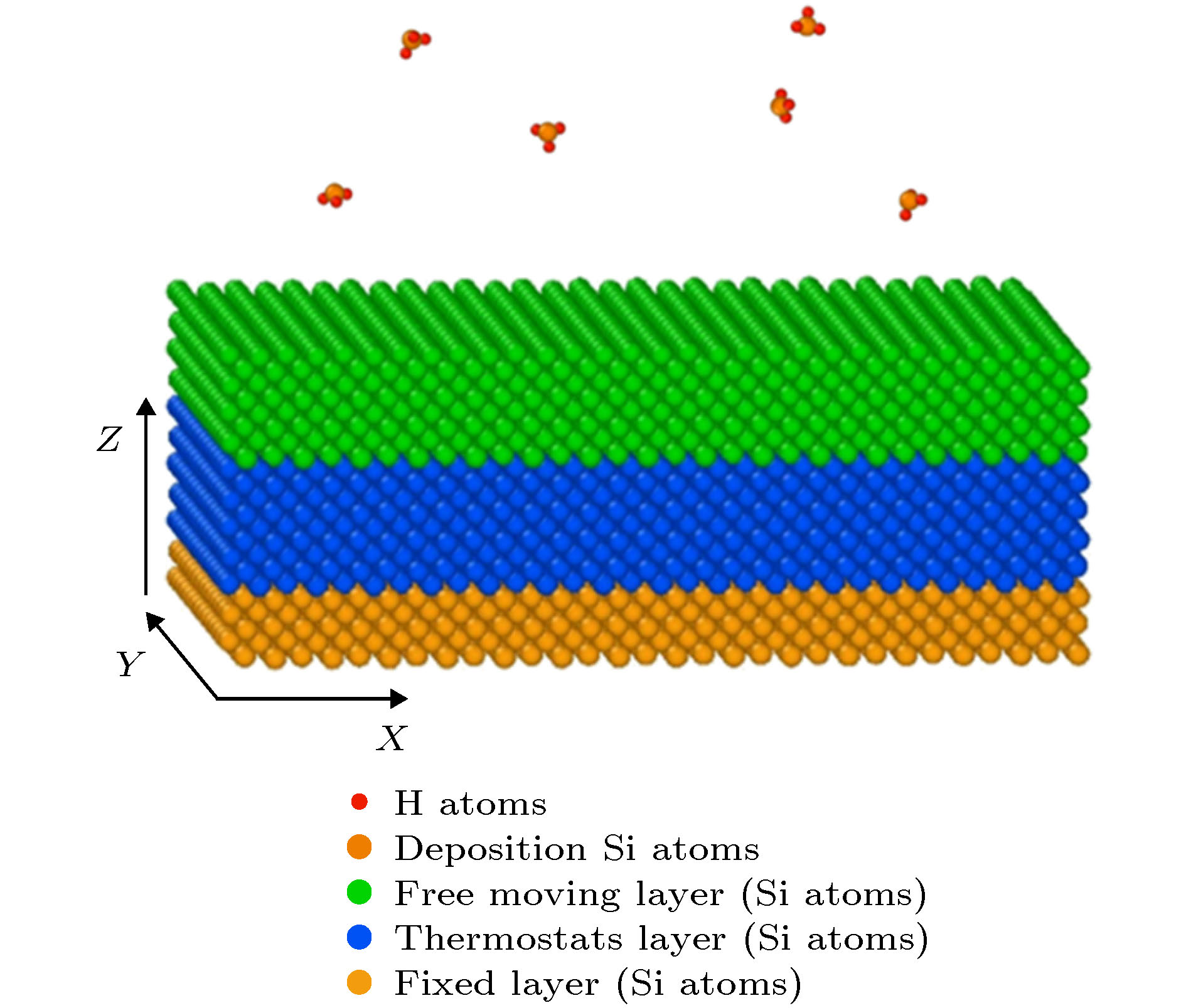
 下载:
下载: