-
非晶合金是原子结构长程无序的亚稳态材料, 具有优异的催化降解性能, 同时也很容易发生晶化, 但晶化对催化降解性能的影响机理目前尚不明确. 本文研究了退火晶化对Fe-Si-B-Cu-Nb工业非晶条带微观结构及其对酸性橙7催化降解性能的影响. 研究发现: 经460—580 ℃退火后, 条带的催化降解性能大幅下降, 其反应速率常数低于0.01 min–1, α-Fe析出相导致其非晶结构的破坏, 降低了羟基自由基的形成速率; 而经过650—700 ℃退火后, 条带的催化降解性能显著提高, 反应速率可提升至退火前的3.77倍, 降解15 min时的脱色率达99.22%, 为退火前的1.12倍, 催化降解性能的提高得益于晶化相与金属化合物间的原电池效应及富集Cu团簇和零价铁之间的置换反应. 本研究揭示了退火晶化对偶氮染料的铁基非晶条带催化降解性能的作用机理, 为利用老化的铁基非晶工业条带处理印染废水、实现“以废治废”, 提供了有益的理论与实验支撑.Amorphous alloys are meta-stable materials with long-range disordered atomic structure, which have excellent catalytic degradation performance and are also susceptible to crystallization, but the mechanism of the effect of crystallization on their catalytic properties has not been clarified. Therefore, the effect of the annealing crystallization process on the microstructure of Fe-Si-B-Cu-Nb industrial amorphous ribbons and their catalytic degradation properties for acid orange 7 are investigated in this work. It is found that the catalytic degradation performance of the ribbons decreases dramatically after having been annealed at 460–580 ℃ , and its reaction rate constant is less than 0.01 min–1. The main reason is the formation of α-Fe precipitation phase in the ribbons after having been annealed at high temperatures and the destruction of the substable amorphous structure. These reduce the rate of hydroxyl radical formation. In contrast, the catalytic degradation performance of the 650–700 ℃ annealed ribbons increases significantly, which increases to 3.77 times the degradation rate of the as-cast ribbons. The decolorization rate of acid orange 7 by the annealed ribbons reaches 99.22% within 15 min, which is 1.12 times that of the as-cast ribbons. The improvement of the catalytic degradation performance is attributed to the primary cell effect between the crystalline phase and the metal compounds and the substitution reaction between the Cu-enriched clusters and zero-valent iron. In this study, the influence mechanism of annealing crystallization on the performance of Fe-Si-B-Cu-Nb industrial amorphous ribbons for degrading azo dyes is revealed, which provides theoretical and experimental support for using aged iron-based amorphous ribbons to purify printing and dyeing waste-water and achieve “purification of waste-water by using alloy waste”.
-
Keywords:
- Fe-based amorphous ribbons /
- industrial ribbons /
- annealed crystallization /
- catalytic degradation
[1] 杨卫明, 刘海顺, 敦超超, 赵玉成, 窦林名 2012 61 106802
 Google Scholar
Google Scholar
Yang W M, Liu H S, Dun C C, Zhao Y C, Dou L M 2012 Acta Phys. Sin. 61 106802
 Google Scholar
Google Scholar
[2] 柳延辉 2017 66 176106
 Google Scholar
Google Scholar
Liu Y H 2017 Acta Phys. Sin. 66 176106
 Google Scholar
Google Scholar
[3] 柯海波, 蒲朕, 张培, 张鹏国, 徐宏扬, 黄火根, 刘天伟, 王英敏 2017 66 176104
 Google Scholar
Google Scholar
Ke H B, Pu Z, Zhang P, Zhang P G, Xu H Y, Huang H G, Liu T W, Wang Y M, 2017 Acta Phys. Sin. 66 176104
 Google Scholar
Google Scholar
[4] Lai L M, Liu T H, Cai X H, Wang M, Zhang S B, Chen W, Guo S F 2021 Scripta Mater 203 114095
 Google Scholar
Google Scholar
[5] 孙奕韬, 王超, 吕玉苗, 胡远超, 罗鹏, 刘明, 咸海杰, 赵德乾, 丁大伟, 孙保安, 潘明祥, 闻平, 白海洋, 柳延辉, 汪卫华 2018 67 126101
 Google Scholar
Google Scholar
Sun Y T, Wang C, Lu Y M, Hu Y C, Luo P, Liu M, Xian H J, Zhao D Q, Ding D W, Sun B A, Pan M X, Wen P, Bai H Y, Liu Y H, Wang W H 2018 Acta Phys. Sin. 67 126101
 Google Scholar
Google Scholar
[6] 姚可夫, 施凌翔, 陈双琴, 邵洋, 陈娜, 贾蓟丽 2018 67 016101
 Google Scholar
Google Scholar
Yao K F, Shi L X, Chen S Q, Shao Y, Chen N, Jia J L 2018 Acta Phys. Sin. 67 016101
 Google Scholar
Google Scholar
[7] Ge Y, Cheng J, Mo J, Xue L, Zhang B, Hong S, Wu Y, Liang X, Zhang X 2024 J. Alloy Compd. 976 173173
 Google Scholar
Google Scholar
[8] Liu H, Jiang Y, Yang D, Jiang Q, Yang W 2023 J. Mat. Res. Technol. 26 3070
 Google Scholar
Google Scholar
[9] Li X, Wang J G, Ke H B, Yang C, Wang W H 2022 Mater. Today Phys. 27 100782
 Google Scholar
Google Scholar
[10] 邵梓桥, 毕恒昌, 谢骁, 万能, 孙立涛 2018 67 167802
 Google Scholar
Google Scholar
Shao Z Q, Bi H C, Xie R, Wan N, Sun L T 2018 Acta Phys. Sin. 67 167802
 Google Scholar
Google Scholar
[11] Qiao J C, Wang Q, Pelletier J M, Kato H, Casalini R, Crespo D, Pineda E, Yao Y, Yang Y 2019 Prog. Mater. Sci. 104 250
 Google Scholar
Google Scholar
[12] Liang S X, Jia Z, Zhang W C, Li X F, Wang W M, Lin H C, Zhang L C 2018 Appl. Catal. B-Environ. 221 108
 Google Scholar
Google Scholar
[13] Zuo M Q, Yi S H, Choi J 2021 J. Environ. Sci. 105 116
 Google Scholar
Google Scholar
[14] Miao F, Wang Q Q, Di S Y, Yun L, Zhou J, Shen B L 2020 J. Mater. Sci. Technol. 53 163
 Google Scholar
Google Scholar
[15] Chen Q, Yan Z C, Zhang H, Zhang L C, Ma H J, Wang W L, Wang W M 2020 Materials 13 3694
 Google Scholar
Google Scholar
[16] Tang Y, Shao Y, Chen N, Yao K F 2015 Rsc Adv. 5 6215
 Google Scholar
Google Scholar
[17] Jia Z, Duan X G, Qin P, Zhang W C, Wang W M, Yang C, Sun H Q, Wang S B, Zhang L C 2017 Adv. Funct. Mat. 27 1702258
 Google Scholar
Google Scholar
[18] Liang S X, Wang X Q, Zhang W C, Liu Y J, Wang W M, Zhang L C 2020 Appl. Mat. Today 19 100543
 Google Scholar
Google Scholar
[19] Wang X Q, Zhang Q Y, Liang S X, Jia Z, Zhang W C, Wang W M, Zhang L C 2020 Catalysts 10 48
 Google Scholar
Google Scholar
[20] Ji L, Chen J W, Zheng Z G, Qiu Z G, Peng S Y, Zhou S H, Zeng D C 2020 J. Phys. Chem. Solids 145 109546
 Google Scholar
Google Scholar
[21] Wang Q Q, Chen M X, Lin P H, Cui Z Q, Chu C L, Shen B L 2018 J. Mat. Chem. A 6 10686
 Google Scholar
Google Scholar
[22] Hou L, Wang Q Q, Fan X D, Miao F, Yang W M, Shen B L 2019 New J. Chem. 43 6126
 Google Scholar
Google Scholar
[23] Chen S Q, Li M, Ji Q M, Chen X, Lan S, Feng T, Yao K F 2021 J. Non-Cryst. Solids 571 121070
 Google Scholar
Google Scholar
[24] Tan L, Wang X Y, Wang S K, Qin X R, Xiao L F, Li C L, Sun S Q, Hu S Q 2023 New J. Chem. 47 11723
 Google Scholar
Google Scholar
[25] Wei J, Zheng Z G, Zhao L, Qiu Z G, Zeng D C 2023 Colloid Surface A 666 131227
 Google Scholar
Google Scholar
[26] Lassoued A, Li J F 2023 J. Phys. Chem. Solids 180 111475
 Google Scholar
Google Scholar
[27] Yang W M, Wang Q Q, Li W Y, Xue L, Liu H S, Zhou J, Mo J Y, Shen B L 2019 Mat. Design 161 136
 Google Scholar
Google Scholar
[28] Zheng K L, Qin X D, Zhu Z W, Zheng S J, Li H L, Fu H M, Zhang H F 2020 J. Mat. Chem. A 8 10855
 Google Scholar
Google Scholar
[29] Luo X K, Li R, Zong J Y, Zhang Y, Li H F, Zhang T 2014 Appl. Surf. Sci. 305 314
 Google Scholar
Google Scholar
[30] Tang M F, Lai L M, Ding D Y, Liu T H, Kang W Z, Guo N, Song B, Guo S F 2022 J. Non-Cryst. Solids 576 121282
 Google Scholar
Google Scholar
[31] Zhao B W, Zhu Z W, Qin X D, Li Z K, Zhang H F 2020 J. Mat. Sci. Technol. 46 88
 Google Scholar
Google Scholar
[32] Qin X D, Zhu Z W, Liu G, Fu H M, Zhang H W, Wang A M, Li H, Zhang H F, 2015 Sci. Rep. 5 18226
 Google Scholar
Google Scholar
[33] Xie S H, Huang P, Kruzic J J, Zeng X, Qian H 2016 Sci. Rep. 6 21947
 Google Scholar
Google Scholar
[34] 陈双琴 2018 博士学位论文 (北京: 清华大学)
Chen S Q 2018 Ph. D Dissertation (Beijing: Tsinghua University
[35] Xie S H, Xie Y L, Jamie J, Gu K M, Yang H P, Deng Y M, Zeng X R 2020 Chemcatchem 12 750
 Google Scholar
Google Scholar
-
图 3 工业非晶条带及其经不同温度退火20 min后降解酸性橙7的紫外光谱图 (a) 工业非晶条带; (b) 460℃; (c) 520 ℃; (d) 580 ℃; (e) 650 ℃; (f) 700 ℃
Fig. 3. UV spectra of industrial amorphous ribbons and their degraded acid orange 7 after annealing at different temperatures for 20 min: (a) Industrial amorphous ribbon; (b) 460℃; (c) 520 ℃; (d) 580 ℃; (e) 650 ℃; (f) 700 ℃.
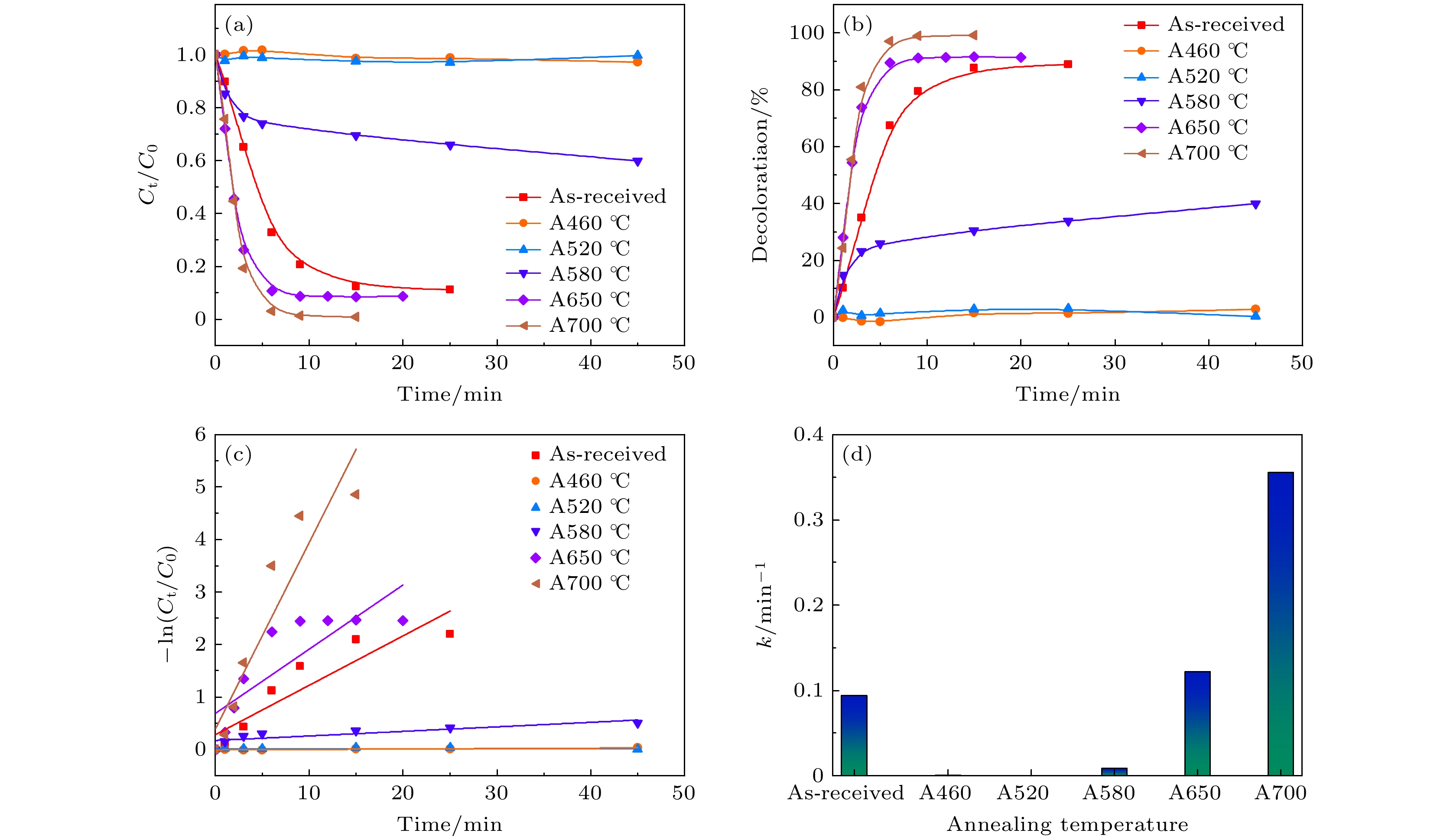
图 4 Fe-Si-B-Cu-Nb工业非晶条带及其经不同温度退火后对酸性橙7的降解 (a) Ct/C0归一曲线; (b)染料脱色率; (c) -ln函数; (d)反应速率常数柱状图
Fig. 4. Degradation of acid orange II processed by Fe-Si-B-Cu-Nb industrial amorphous ribbons in the as-cast state and after different annealing temperatures: (a) Ct/C0 normalized graph; (b) dye decolorization rate graph; (c) variation of the -ln function graph; (d) histogram of reaction rate constants.
表 1 用于偶氮染料催化降解的典型非晶合金比较
Table 1. Comparison of typical amorphous alloys for catalytic degradation of azo dyes.
合金成分 样品
状态染料种类 反应速率常数(kobs) 循环次数 参考
文献Fe83Si5B8P4 条带 亚甲基蓝 0.514 — [13] (Fe78Si9B13)99Zr1 条带 亚甲基蓝 0.24 — [14] Fe84B16 条带 直接蓝6 0.110 — [16] Fe78Si9B13 条带 甲基蓝 0.085 — [17] Fe78Si11B9P2 条带 酸性橙7 0.082 17 [20] Fe80P13C7 条带 亚甲基蓝 0.56 23 [21] Cu46Zr44.5Al7.5Gd2 条带 酸性橙7 0.48 80 [28] Mg65Cu25Y10 粉末 直接蓝6 0.585 — [29] Co65Mo15B20 非晶丝 直接蓝6 2.31 20 [30] Cu47.5Zr46Al6.5 条带 酸性橙7 0.165 10 [31] Co78Si8B14 粉末 酸性橙7 — 8 [32] FeSiBCuNb 条带 酸性橙7 0.094 50 本工作 FeSiBCuNb (700℃退火后) 条带 酸性橙7 0.36 — 本工作 表 2 α-Fe晶粒尺寸计算结果
Table 2. Calculation results of α-Fe grain size.
温度/℃ 460 520 580 650 700 βhkl/(°) 0.80 0.71 0.66 0.27 0.21 Dhkl/nm 11.54 12.68 13.72 33.74 42.52 (hkl) (220) (220) (220) (220) (220) -
[1] 杨卫明, 刘海顺, 敦超超, 赵玉成, 窦林名 2012 61 106802
 Google Scholar
Google Scholar
Yang W M, Liu H S, Dun C C, Zhao Y C, Dou L M 2012 Acta Phys. Sin. 61 106802
 Google Scholar
Google Scholar
[2] 柳延辉 2017 66 176106
 Google Scholar
Google Scholar
Liu Y H 2017 Acta Phys. Sin. 66 176106
 Google Scholar
Google Scholar
[3] 柯海波, 蒲朕, 张培, 张鹏国, 徐宏扬, 黄火根, 刘天伟, 王英敏 2017 66 176104
 Google Scholar
Google Scholar
Ke H B, Pu Z, Zhang P, Zhang P G, Xu H Y, Huang H G, Liu T W, Wang Y M, 2017 Acta Phys. Sin. 66 176104
 Google Scholar
Google Scholar
[4] Lai L M, Liu T H, Cai X H, Wang M, Zhang S B, Chen W, Guo S F 2021 Scripta Mater 203 114095
 Google Scholar
Google Scholar
[5] 孙奕韬, 王超, 吕玉苗, 胡远超, 罗鹏, 刘明, 咸海杰, 赵德乾, 丁大伟, 孙保安, 潘明祥, 闻平, 白海洋, 柳延辉, 汪卫华 2018 67 126101
 Google Scholar
Google Scholar
Sun Y T, Wang C, Lu Y M, Hu Y C, Luo P, Liu M, Xian H J, Zhao D Q, Ding D W, Sun B A, Pan M X, Wen P, Bai H Y, Liu Y H, Wang W H 2018 Acta Phys. Sin. 67 126101
 Google Scholar
Google Scholar
[6] 姚可夫, 施凌翔, 陈双琴, 邵洋, 陈娜, 贾蓟丽 2018 67 016101
 Google Scholar
Google Scholar
Yao K F, Shi L X, Chen S Q, Shao Y, Chen N, Jia J L 2018 Acta Phys. Sin. 67 016101
 Google Scholar
Google Scholar
[7] Ge Y, Cheng J, Mo J, Xue L, Zhang B, Hong S, Wu Y, Liang X, Zhang X 2024 J. Alloy Compd. 976 173173
 Google Scholar
Google Scholar
[8] Liu H, Jiang Y, Yang D, Jiang Q, Yang W 2023 J. Mat. Res. Technol. 26 3070
 Google Scholar
Google Scholar
[9] Li X, Wang J G, Ke H B, Yang C, Wang W H 2022 Mater. Today Phys. 27 100782
 Google Scholar
Google Scholar
[10] 邵梓桥, 毕恒昌, 谢骁, 万能, 孙立涛 2018 67 167802
 Google Scholar
Google Scholar
Shao Z Q, Bi H C, Xie R, Wan N, Sun L T 2018 Acta Phys. Sin. 67 167802
 Google Scholar
Google Scholar
[11] Qiao J C, Wang Q, Pelletier J M, Kato H, Casalini R, Crespo D, Pineda E, Yao Y, Yang Y 2019 Prog. Mater. Sci. 104 250
 Google Scholar
Google Scholar
[12] Liang S X, Jia Z, Zhang W C, Li X F, Wang W M, Lin H C, Zhang L C 2018 Appl. Catal. B-Environ. 221 108
 Google Scholar
Google Scholar
[13] Zuo M Q, Yi S H, Choi J 2021 J. Environ. Sci. 105 116
 Google Scholar
Google Scholar
[14] Miao F, Wang Q Q, Di S Y, Yun L, Zhou J, Shen B L 2020 J. Mater. Sci. Technol. 53 163
 Google Scholar
Google Scholar
[15] Chen Q, Yan Z C, Zhang H, Zhang L C, Ma H J, Wang W L, Wang W M 2020 Materials 13 3694
 Google Scholar
Google Scholar
[16] Tang Y, Shao Y, Chen N, Yao K F 2015 Rsc Adv. 5 6215
 Google Scholar
Google Scholar
[17] Jia Z, Duan X G, Qin P, Zhang W C, Wang W M, Yang C, Sun H Q, Wang S B, Zhang L C 2017 Adv. Funct. Mat. 27 1702258
 Google Scholar
Google Scholar
[18] Liang S X, Wang X Q, Zhang W C, Liu Y J, Wang W M, Zhang L C 2020 Appl. Mat. Today 19 100543
 Google Scholar
Google Scholar
[19] Wang X Q, Zhang Q Y, Liang S X, Jia Z, Zhang W C, Wang W M, Zhang L C 2020 Catalysts 10 48
 Google Scholar
Google Scholar
[20] Ji L, Chen J W, Zheng Z G, Qiu Z G, Peng S Y, Zhou S H, Zeng D C 2020 J. Phys. Chem. Solids 145 109546
 Google Scholar
Google Scholar
[21] Wang Q Q, Chen M X, Lin P H, Cui Z Q, Chu C L, Shen B L 2018 J. Mat. Chem. A 6 10686
 Google Scholar
Google Scholar
[22] Hou L, Wang Q Q, Fan X D, Miao F, Yang W M, Shen B L 2019 New J. Chem. 43 6126
 Google Scholar
Google Scholar
[23] Chen S Q, Li M, Ji Q M, Chen X, Lan S, Feng T, Yao K F 2021 J. Non-Cryst. Solids 571 121070
 Google Scholar
Google Scholar
[24] Tan L, Wang X Y, Wang S K, Qin X R, Xiao L F, Li C L, Sun S Q, Hu S Q 2023 New J. Chem. 47 11723
 Google Scholar
Google Scholar
[25] Wei J, Zheng Z G, Zhao L, Qiu Z G, Zeng D C 2023 Colloid Surface A 666 131227
 Google Scholar
Google Scholar
[26] Lassoued A, Li J F 2023 J. Phys. Chem. Solids 180 111475
 Google Scholar
Google Scholar
[27] Yang W M, Wang Q Q, Li W Y, Xue L, Liu H S, Zhou J, Mo J Y, Shen B L 2019 Mat. Design 161 136
 Google Scholar
Google Scholar
[28] Zheng K L, Qin X D, Zhu Z W, Zheng S J, Li H L, Fu H M, Zhang H F 2020 J. Mat. Chem. A 8 10855
 Google Scholar
Google Scholar
[29] Luo X K, Li R, Zong J Y, Zhang Y, Li H F, Zhang T 2014 Appl. Surf. Sci. 305 314
 Google Scholar
Google Scholar
[30] Tang M F, Lai L M, Ding D Y, Liu T H, Kang W Z, Guo N, Song B, Guo S F 2022 J. Non-Cryst. Solids 576 121282
 Google Scholar
Google Scholar
[31] Zhao B W, Zhu Z W, Qin X D, Li Z K, Zhang H F 2020 J. Mat. Sci. Technol. 46 88
 Google Scholar
Google Scholar
[32] Qin X D, Zhu Z W, Liu G, Fu H M, Zhang H W, Wang A M, Li H, Zhang H F, 2015 Sci. Rep. 5 18226
 Google Scholar
Google Scholar
[33] Xie S H, Huang P, Kruzic J J, Zeng X, Qian H 2016 Sci. Rep. 6 21947
 Google Scholar
Google Scholar
[34] 陈双琴 2018 博士学位论文 (北京: 清华大学)
Chen S Q 2018 Ph. D Dissertation (Beijing: Tsinghua University
[35] Xie S H, Xie Y L, Jamie J, Gu K M, Yang H P, Deng Y M, Zeng X R 2020 Chemcatchem 12 750
 Google Scholar
Google Scholar
计量
- 文章访问数: 8461
- PDF下载量: 92
- 被引次数: 0













 下载:
下载: