-
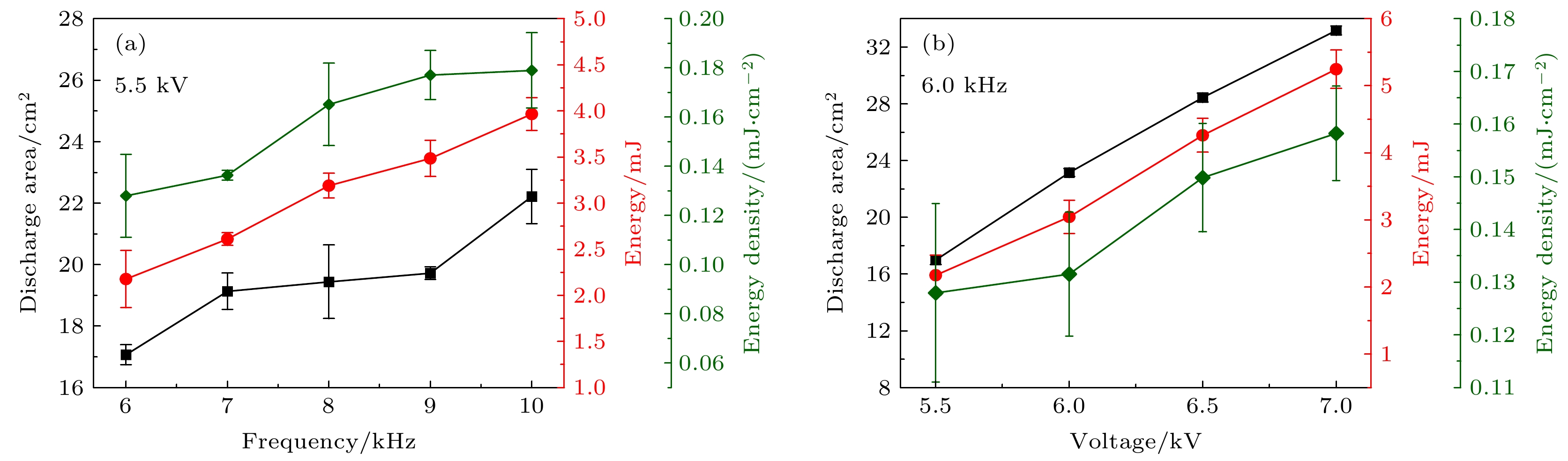
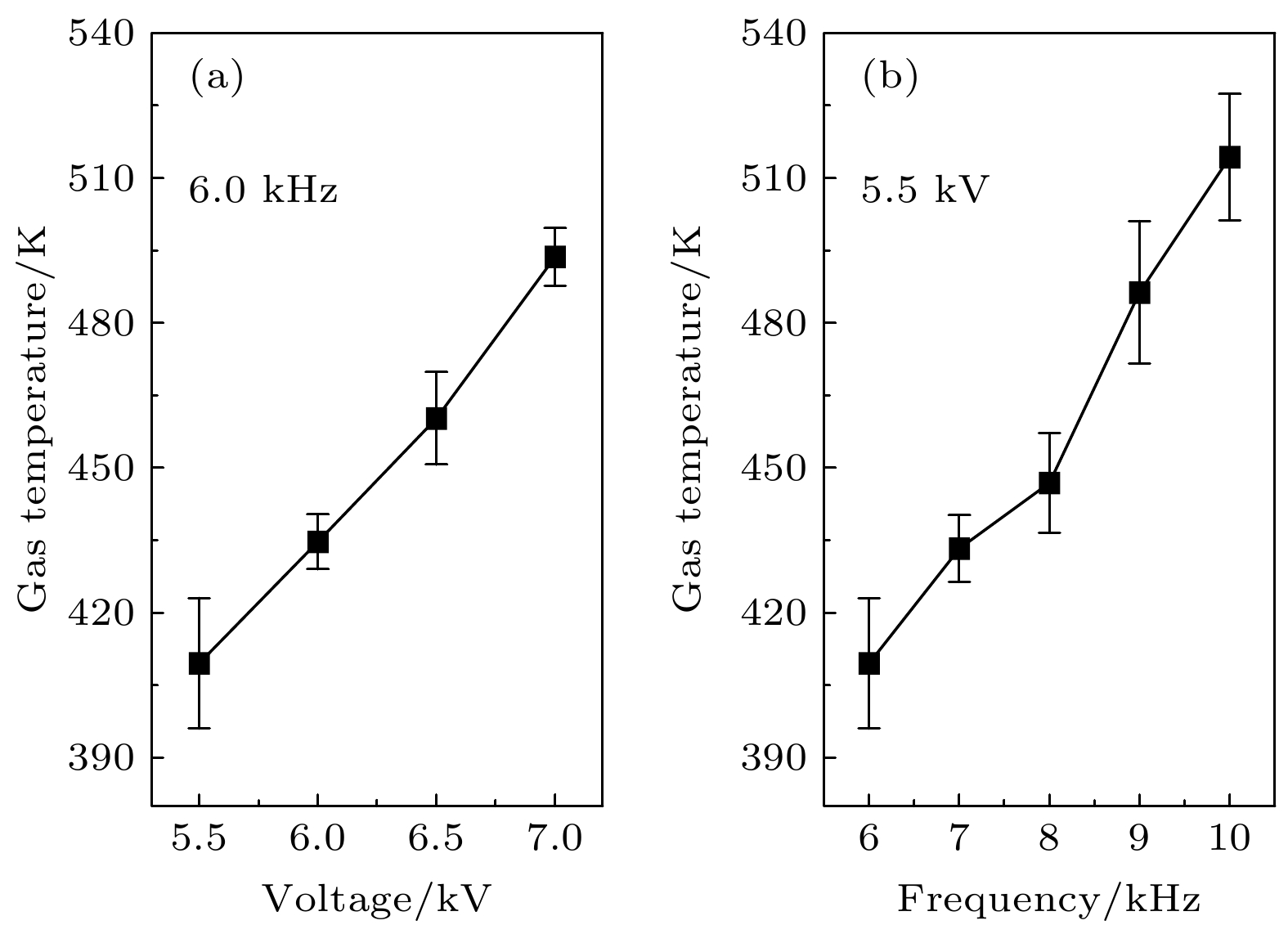
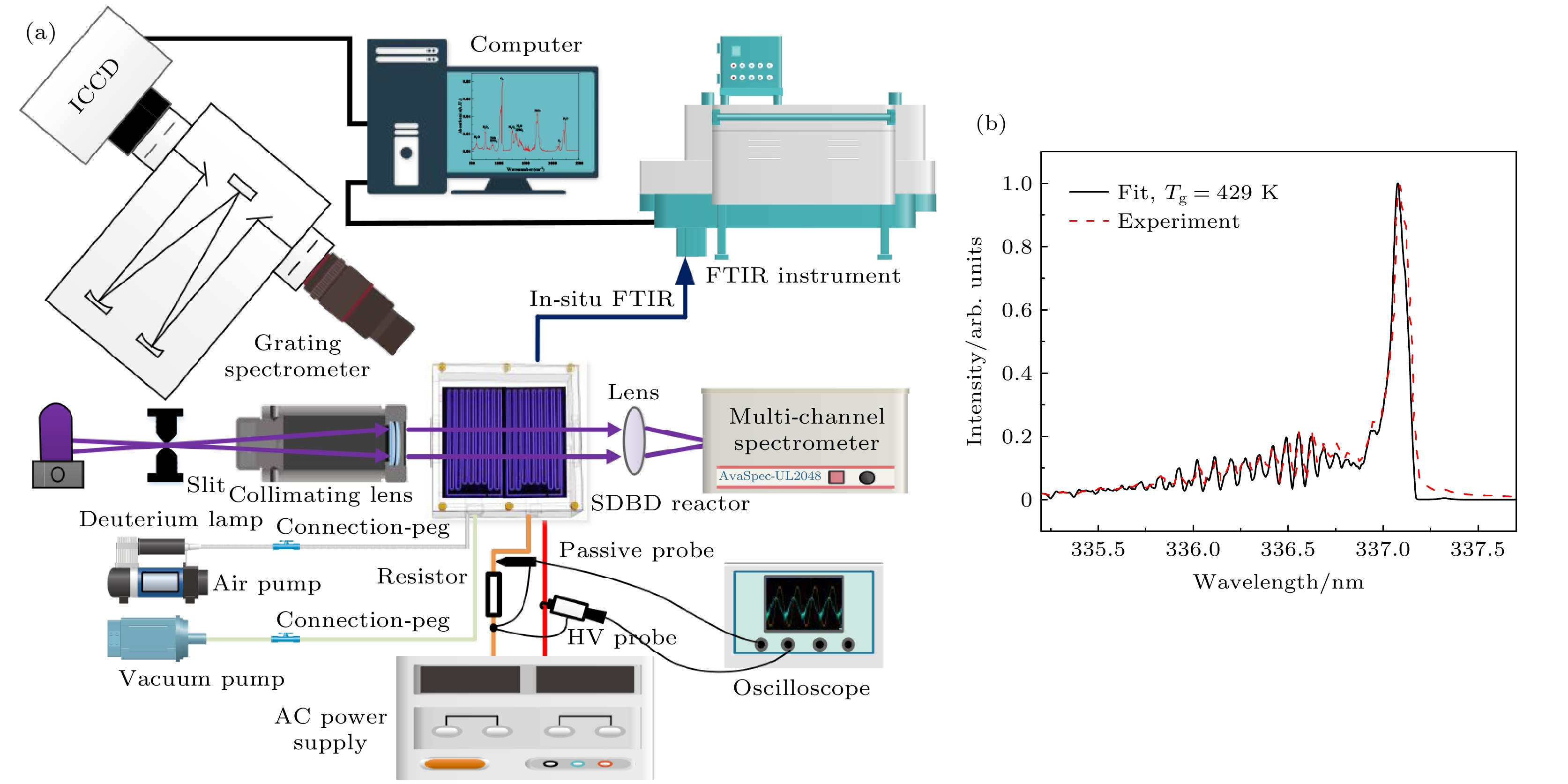
为了深入了解大气压空气等离子体气态产物的变化的物理-化学机理, 本文以沿面介质阻挡放电为研究对象, 采用傅里叶红外光谱和紫外吸收光谱法原位测量了不同电压和频率下特征产物(一氧化氮(NO)和臭氧(O3))浓度的动态变化过程, 并基于李萨如图和放电图像计算了等离子体的真实能量密度, 通过拟合氮分子第二正带的发射光谱得到了气体温度. 结果表明, 在更高的电压和频率下, O3的吸光度更低而NO的吸光度更高, 还会加速产物从包含O3的状态转化为无O3的状态, 此时真实能量密度和气体温度也更高. 通过分析真实能量密度和气体温度对特征产物的生成和猝灭化学反应的影响, 揭示了产物变化的微观机理. 分析表明, O3消失主要是由于O和O与O2的激发态粒子以及NO对O3的猝灭导致, 其消失速度随着能量密度和气体温度的升高而加快. 反观NO, 气体温度的升高能显著提高其生成反应的速率, 并抑制其解离速率. 这有助于更快地生成大量NO, 加速其对O3的猝灭进程, 这也是O3消失越来越快的外因.To gain an insight into the interaction mechanism among the gaseous products of atmospheric pressure air plasma, a surface dielectric barrier discharge is used as a study object. The dynamic processes of characteristic products (nitric oxide NO and ozone O3) are measured by in-situ Fourier infrared spectroscopy and UV absorption spectroscopy. The real energy density of the plasma is calculated by Lissajous figure and ICCD optical image. The gas temperature is obtained by fitting the emission spectrum of the second positive band of the nitrogen molecule. The results show that the real energy density and gas temperature are highly positively correlated with the applied voltage and frequency. Higher applied voltages and frequencies can lead to lower peak absorbance of O3 and higher absorbance of NO, and accelerate the conversion of the products from O3-containing state into O3-free state. The microscopic mechanism of the product change is revealed by analyzing the effects of the real energy density and gas temperature on the major generation and quenching chemical reactions of the characteristic products. The analysis points out that there are two major reasons for the disappearance of O3, i.e. the quenching effect of O and O/O2 excited state particles on O3 and the quenching effect of NO on O3. And the mechanism that the disappearance of O3 accelerates with the increase of energy density and gas temperature, is as follows. The increase of real energy density means that the energy injected into the discharge region is enhanced, which intensifies the collision reaction, thereby producing more energetic electrons and reactive oxygen and nitrogen particles. Since the discharge cavity is gas-tight, the rapid generation of O leads to a rapid increase in the ratio of O to O2, which accelerates the decomposition of O3; besides, the gas temperature is raised due to the intensification of the collision reaction. Whereas the gas temperature can change the rate coefficients of the chemical reactions involving the excited state particles of nitrogen and oxygen to regulate the production and quenching of the products. The increase of gas temperature has a negative effect on O3. The higher the gas temperature, the lower the rate of O3 generation reaction is but the higher the rate of dissociation, which is thought to be the endogenous cause of the rapid disappearance of O3. In contrast, the gas temperature rising can significantly elevate the reaction rate of NO production and reduces its dissociation rate. This contributes to the faster production of massive NO, resulting in an accelerated quenching process of NO to O3, which can be considered as the exogenous cause of the rapid disappearance of O3. In a word, the present study contributes to a better understanding of the physico-chemical process in atmospheric pressure low-temperature plasma.
-
Keywords:
- surface dielectric barrier discharge /
- real energy density /
- gas temperature /
- chemical products
[1] Liu K, Hu Y, Lei J 2017 Phys. Plasmas 24 103513
 Google Scholar
Google Scholar
[2] 商克峰, 王美威, 鲁娜, 姜楠, 李杰, 吴彦 2021 高电压技术 47 353
 Google Scholar
Google Scholar
Shang K F, Wang M W, Lu N, Jiang N, Li J, Wu Y 2021 High Volt. Eng. 47 353
 Google Scholar
Google Scholar
[3] 王兴生, 马彦明, 高勋, 林景全 2020 69 029502
 Google Scholar
Google Scholar
Wang X S, Ma Y M, Gao X, Lin J Q 2020 Acta Phys. Sin. 69 029502
 Google Scholar
Google Scholar
[4] Liu K, Zheng Z F, Liu S T, Hu Y Y 2019 Plasma Chem. Plasma Process. 39 1255
 Google Scholar
Google Scholar
[5] 高书涵, 王绪成, 张远涛 2020 69 115204
 Google Scholar
Google Scholar
Gao S H, Wang X C, Zhang Y T 2020 Acta Phys. Sin. 69 115204
 Google Scholar
Google Scholar
[6] Pang B L, Liu Z J, Zhang H Y, Wang S T, Gao Y T, Xu D H, Liu D X, Kong M G 2022 Plasma Process. Polym. 19 e2100079
 Google Scholar
Google Scholar
[7] Zhao Z L, Wang W C, Yang D Z, Zhou X F, Yuan H 2019 IEEE Trans. Plasma Sci. 47 4219
 Google Scholar
Google Scholar
[8] Peng B F, Jiang N, Shang K F, Lu N, Li J, Wu Y 2022 J. Phys. D Appl. Phys. 55 265202
 Google Scholar
Google Scholar
[9] Jiang N, Kong X Q, Lu X L, Peng B F, Liu Z Y, Li J, Shang K F, Lu N, Wu Y 2022 J. Clean. Prod. 332 129998
 Google Scholar
Google Scholar
[10] Dascalu A, Pohoata V, Shimizu K, Sirghi L 2021 Plasma Chem. Plasma Process. 41 389
 Google Scholar
Google Scholar
[11] Park S, Choe W, Jo C 2018 Chem. Eng. J. 352 1014
 Google Scholar
Google Scholar
[12] Douat C, Hubner S, Engeln R, Benedikt J 2016 Plasma Sources Sci. Technol. 25 025027
 Google Scholar
Google Scholar
[13] Qin H B, Qiu H J, He S T, Hong B X, Liu K, Lou F X, Li M C, Hu P, Kong X H, Song Y J, Liu Y C, Pu M F, Han P J, Li M Z, An X P, Song L H, Tong Y G, Fan H H, Wang R X 2022 J. Hazard. Mater. 430 128414
 Google Scholar
Google Scholar
[14] Wang S T, Liu Z J, Pang B L, Gao Y T, Luo S T, Li Q S, Chen H L, Kong M G 2022 Appl. Phys. Lett. 121 144101
 Google Scholar
Google Scholar
[15] Shimizu T, Ikehara 2017 J. Phys. D Appl. Phys. 50 503001
 Google Scholar
Google Scholar
[16] Shimizu T, Sakiyama Y, Graves D B, Zimmermann J L, Morfill G E 2012 New J. Phys. 14 103028
 Google Scholar
Google Scholar
[17] Pavlovich M J, Clark D S, Graves D B 2014 Plasma Sources Sci. Technol. 23 065036
 Google Scholar
Google Scholar
[18] Xi W, Wang W, Liu Z J, Wang Z F, Guo L, Wang X H, Rong M Z, Liu D X 2020 Plasma Sources Sci. Technol. 29 095013
 Google Scholar
Google Scholar
[19] Waskow A, Ibba L, Leftley M, Howling A, Ambrico P F, Furno I 2021 Int. J. Mol. Sci. 22 11540
 Google Scholar
Google Scholar
[20] 万海容, 郝艳捧, 房强, 苏恒炜, 阳林, 李立浧 2020 69 145203
 Google Scholar
Google Scholar
Wan H R, Hao Y P, Fang Q, Su H W, Yang L, Li L C 2020 Acta Phys. Sin. 69 145203
 Google Scholar
Google Scholar
[21] Yuan H, Wang W C, Yang D Z, Zhao Z L, Zhang L, Wang S 2017 Plasma Sci. Technol. 19 125401
 Google Scholar
Google Scholar
[22] Liu K, Ren W, Ran C F, Zhou R S, Tang W B, Zhou R W, Yang Z H, Ostrikov K 2021 J. Phys. D:Appl. Phys. 54 065201
 Google Scholar
Google Scholar
[23] Liu K, Xia H T, Yang M H, Geng W Q, Zuo J, Ostrikov K 2022 Vacuum 198 110901
 Google Scholar
Google Scholar
[24] Liu K, Duan Q S, Zheng Z F, Zhou R S, Zhou R W, Tang W B, Cullen P, Ostrikov K 2021 Plasma Process. Polym. 18 2100016
 Google Scholar
Google Scholar
[25] Liu K, Lei J, Zheng Z, Zhu Z, Liu S 2018 Appl. Surf. Sci. 458 183
 Google Scholar
Google Scholar
[26] 高坤, 弓丹丹, 刘仁静, 苏泽华, 贾鹏英, 李雪辰 2020 光谱学与光谱分析 40 461
 Google Scholar
Google Scholar
Gao K, Gong D D, Liu R J, Su Z H, Jia P Y, Li X C 2020 Spectrosc. Spect. Anal. 40 461
 Google Scholar
Google Scholar
[27] Sakiyama Y, Graves D B, Chang H W, Shimizu T, Morfill G E 2012 J. Phys. D Appl. Phys. 45 425201
 Google Scholar
Google Scholar
[28] Park G Y, Park S J, Choi M Y, Koo I G, Byun J H, Hong J W, Sim J Y, Collins G J, Lee J K 2012 Plasma Sources Sci. Technol. 21 043001
 Google Scholar
Google Scholar
[29] 李森, 王小兵, 马婷婷, 李栋, 戴健男 2021 真空科学与技术学报 41 619
 Google Scholar
Google Scholar
Li S, Wang X B, Ma T T, Li D, Dai J N 2021 Chin. J. Vac. Sci. Technol. 41 619
 Google Scholar
Google Scholar
[30] Yin S E, Sun B M, Gao X D, Xiao H P 2009 Plasma Chem. Plasma Process. 29 421
 Google Scholar
Google Scholar
[31] Zhou X F, Zhao Z L, Liang J P, Yuan H, Wang W C, Yang D Z 2019 Plasma Process. Polym. 16 e1900001
 Google Scholar
Google Scholar
[32] 陈忠琪, 钟安, 戴栋, 宁文军 2022 71 165201
 Google Scholar
Google Scholar
Chen Z Q, Zhong A, Dai D, Ning W J 2022 Acta Phys. Sin. 71 165201
 Google Scholar
Google Scholar
[33] Eliasson B, Hirth M, Kogelschatz U 1987 J. Phys. D Appl. Phys. 20 1421
 Google Scholar
Google Scholar
[34] Vervloessem E, Aghaei M, Jardali F, Hafezkhiabani N, Bogaerts A 2020 ACS Sustainale Chem. Eng. 8 9711
 Google Scholar
Google Scholar
[35] Gordillo-Vazquez F J 2008 J. Phys. D Appl. Phys. 41 234016
 Google Scholar
Google Scholar
[36] Kossyi I A, Kostinsky A Y, Matveyev A A, Silakov V P 1992 Plasma Sources Sci. Technol. 1 207
 Google Scholar
Google Scholar
-
图 4 SDBD放电图像 (a) 6 kHz, 5.5 kV; (b) 6 kHz, 6.0 kV; (c) 6 kHz, 6.5 kV; (d) 6 kHz, 7.0 kV; (e) 7 kHz, 5.5 kV; (f) 8 kHz, 5.5 kV; (g) 9 kHz, 5.5 kV; (h) 10 kHz, 5.5 kV
Fig. 4. SDBD discharge images: (a) 6 kHz, 5.5 kV; (b) 6 kHz, 6.0 kV; (c) 6 kHz, 6.5 kV; (d) 6 kHz, 7.0 kV; (e) 7 kHz, 5.5 kV; (f) 8 kHz, 5.5 kV; (g) 9 kHz, 5.5 kV; (h) 10 kHz, 5.5 kV.
表 1 FTIR中不同物质对应的吸收峰波数
Table 1. Absorption peak wavenumber of differentspecies in FTIR.
化学产物 波数/cm–1 N2O 589, 1285, 2224, 2237 N2O5 743, 880, 1247, 1355, 1720 HNO3 762, 896, 1313, 1341, 1700 O3 1030, 1043, 1055, 2098, 2121 NO2 1600, 1621, 1627 NO 1876 表 2 空气放电中的主要化学反应
Table 2. Main chemical reactions in air discharge.
化学反应 速率系数k 编号 文献 e + N2 → N(2D) + N + e 3.99 × 10–17 ε2.24 exp(–9.10/ε) R1 [27] e + N2 → N2(v) + e BOLSIG+ R2 [34] e + O2 → O + O + e 2.03 × 10–14 ε–0.10 exp(–8.47/ε) R3 [27] e + O2 →O(1D) + O + e 1.82 × 10–14 ε–0.13 exp(–10.70/ε) R4 [27] e + O2 → O2(a) + e 1.04 × 10–15 exp(–2.59/ε) R5 [27] O + O2+ N2 → O3 + N2 5.8 × 10–34 × (300/Tg)2.8 R6 [35] O + O2+ O2 → O3 + O2 7.6 × 10–34 × (300/Tg)1.9 R7 [35] O + O3 → O2 + O2 2 × 10–11 exp(–2300/Tg) R8 [35] O + O3 → O2 + O2(a) 2.0 × 10–11 exp(–2280/Tg) R9 [34] O2(a) + O3 → O2 + O2 + O(1D) 5.20 × 10–11 exp(–2480/Tg) R10 [35] O2(a) + O3 → O + O2 + O2 5.20 × 10–11 exp(–2480/Tg) R11 [35] O(1D) + O3 → O2 + O + O 1.20 × 10–10 R12 [36] NO + O3 → O2 + NO2 4.30 × 10–12 exp(–1560/Tg) R13 [36] O + NO2 → NO + O2 9.10 × 10–12 × (Tg/300)0.18 R14 [35] O + N2(v) → N + NO 3.01 × 10–10 exp(–38370/Tg) R15 [34] N + O3 → NO + O2 5.00 × 10–12 exp(–650/Tg) R16 [34] N + O2 → O + NO 1.0 × 10–11 × exp(–3473/Tg) R17 [35] N + O2(a) → NO + O 2.00 × 10–14 exp(–600/Tg) R18 [35] N(2D) + O2 → NO + O 1.50 × 10–12 exp(Tg/300)0.5 R19 [36] N(2D) + N2O → N2 + NO 1.50 × 10–17 exp(–570/Tg) R20 [27] N(2D) + O2 →NO + O(1D) 6.00 × 10–12 exp(Tg/300)0.5 R21 [36] O + NO + N2 → NO2 + N2 1.20 × 10–31 × (300/Tg)1.7 R22 [35] O + NO + O2 → NO2 + O2 9.36 × 10–32 × (300/Tg)1.7 R23 [35] 注: 二元反应和三元反应的速率系数单位分别为m3/s, m6/s. -
[1] Liu K, Hu Y, Lei J 2017 Phys. Plasmas 24 103513
 Google Scholar
Google Scholar
[2] 商克峰, 王美威, 鲁娜, 姜楠, 李杰, 吴彦 2021 高电压技术 47 353
 Google Scholar
Google Scholar
Shang K F, Wang M W, Lu N, Jiang N, Li J, Wu Y 2021 High Volt. Eng. 47 353
 Google Scholar
Google Scholar
[3] 王兴生, 马彦明, 高勋, 林景全 2020 69 029502
 Google Scholar
Google Scholar
Wang X S, Ma Y M, Gao X, Lin J Q 2020 Acta Phys. Sin. 69 029502
 Google Scholar
Google Scholar
[4] Liu K, Zheng Z F, Liu S T, Hu Y Y 2019 Plasma Chem. Plasma Process. 39 1255
 Google Scholar
Google Scholar
[5] 高书涵, 王绪成, 张远涛 2020 69 115204
 Google Scholar
Google Scholar
Gao S H, Wang X C, Zhang Y T 2020 Acta Phys. Sin. 69 115204
 Google Scholar
Google Scholar
[6] Pang B L, Liu Z J, Zhang H Y, Wang S T, Gao Y T, Xu D H, Liu D X, Kong M G 2022 Plasma Process. Polym. 19 e2100079
 Google Scholar
Google Scholar
[7] Zhao Z L, Wang W C, Yang D Z, Zhou X F, Yuan H 2019 IEEE Trans. Plasma Sci. 47 4219
 Google Scholar
Google Scholar
[8] Peng B F, Jiang N, Shang K F, Lu N, Li J, Wu Y 2022 J. Phys. D Appl. Phys. 55 265202
 Google Scholar
Google Scholar
[9] Jiang N, Kong X Q, Lu X L, Peng B F, Liu Z Y, Li J, Shang K F, Lu N, Wu Y 2022 J. Clean. Prod. 332 129998
 Google Scholar
Google Scholar
[10] Dascalu A, Pohoata V, Shimizu K, Sirghi L 2021 Plasma Chem. Plasma Process. 41 389
 Google Scholar
Google Scholar
[11] Park S, Choe W, Jo C 2018 Chem. Eng. J. 352 1014
 Google Scholar
Google Scholar
[12] Douat C, Hubner S, Engeln R, Benedikt J 2016 Plasma Sources Sci. Technol. 25 025027
 Google Scholar
Google Scholar
[13] Qin H B, Qiu H J, He S T, Hong B X, Liu K, Lou F X, Li M C, Hu P, Kong X H, Song Y J, Liu Y C, Pu M F, Han P J, Li M Z, An X P, Song L H, Tong Y G, Fan H H, Wang R X 2022 J. Hazard. Mater. 430 128414
 Google Scholar
Google Scholar
[14] Wang S T, Liu Z J, Pang B L, Gao Y T, Luo S T, Li Q S, Chen H L, Kong M G 2022 Appl. Phys. Lett. 121 144101
 Google Scholar
Google Scholar
[15] Shimizu T, Ikehara 2017 J. Phys. D Appl. Phys. 50 503001
 Google Scholar
Google Scholar
[16] Shimizu T, Sakiyama Y, Graves D B, Zimmermann J L, Morfill G E 2012 New J. Phys. 14 103028
 Google Scholar
Google Scholar
[17] Pavlovich M J, Clark D S, Graves D B 2014 Plasma Sources Sci. Technol. 23 065036
 Google Scholar
Google Scholar
[18] Xi W, Wang W, Liu Z J, Wang Z F, Guo L, Wang X H, Rong M Z, Liu D X 2020 Plasma Sources Sci. Technol. 29 095013
 Google Scholar
Google Scholar
[19] Waskow A, Ibba L, Leftley M, Howling A, Ambrico P F, Furno I 2021 Int. J. Mol. Sci. 22 11540
 Google Scholar
Google Scholar
[20] 万海容, 郝艳捧, 房强, 苏恒炜, 阳林, 李立浧 2020 69 145203
 Google Scholar
Google Scholar
Wan H R, Hao Y P, Fang Q, Su H W, Yang L, Li L C 2020 Acta Phys. Sin. 69 145203
 Google Scholar
Google Scholar
[21] Yuan H, Wang W C, Yang D Z, Zhao Z L, Zhang L, Wang S 2017 Plasma Sci. Technol. 19 125401
 Google Scholar
Google Scholar
[22] Liu K, Ren W, Ran C F, Zhou R S, Tang W B, Zhou R W, Yang Z H, Ostrikov K 2021 J. Phys. D:Appl. Phys. 54 065201
 Google Scholar
Google Scholar
[23] Liu K, Xia H T, Yang M H, Geng W Q, Zuo J, Ostrikov K 2022 Vacuum 198 110901
 Google Scholar
Google Scholar
[24] Liu K, Duan Q S, Zheng Z F, Zhou R S, Zhou R W, Tang W B, Cullen P, Ostrikov K 2021 Plasma Process. Polym. 18 2100016
 Google Scholar
Google Scholar
[25] Liu K, Lei J, Zheng Z, Zhu Z, Liu S 2018 Appl. Surf. Sci. 458 183
 Google Scholar
Google Scholar
[26] 高坤, 弓丹丹, 刘仁静, 苏泽华, 贾鹏英, 李雪辰 2020 光谱学与光谱分析 40 461
 Google Scholar
Google Scholar
Gao K, Gong D D, Liu R J, Su Z H, Jia P Y, Li X C 2020 Spectrosc. Spect. Anal. 40 461
 Google Scholar
Google Scholar
[27] Sakiyama Y, Graves D B, Chang H W, Shimizu T, Morfill G E 2012 J. Phys. D Appl. Phys. 45 425201
 Google Scholar
Google Scholar
[28] Park G Y, Park S J, Choi M Y, Koo I G, Byun J H, Hong J W, Sim J Y, Collins G J, Lee J K 2012 Plasma Sources Sci. Technol. 21 043001
 Google Scholar
Google Scholar
[29] 李森, 王小兵, 马婷婷, 李栋, 戴健男 2021 真空科学与技术学报 41 619
 Google Scholar
Google Scholar
Li S, Wang X B, Ma T T, Li D, Dai J N 2021 Chin. J. Vac. Sci. Technol. 41 619
 Google Scholar
Google Scholar
[30] Yin S E, Sun B M, Gao X D, Xiao H P 2009 Plasma Chem. Plasma Process. 29 421
 Google Scholar
Google Scholar
[31] Zhou X F, Zhao Z L, Liang J P, Yuan H, Wang W C, Yang D Z 2019 Plasma Process. Polym. 16 e1900001
 Google Scholar
Google Scholar
[32] 陈忠琪, 钟安, 戴栋, 宁文军 2022 71 165201
 Google Scholar
Google Scholar
Chen Z Q, Zhong A, Dai D, Ning W J 2022 Acta Phys. Sin. 71 165201
 Google Scholar
Google Scholar
[33] Eliasson B, Hirth M, Kogelschatz U 1987 J. Phys. D Appl. Phys. 20 1421
 Google Scholar
Google Scholar
[34] Vervloessem E, Aghaei M, Jardali F, Hafezkhiabani N, Bogaerts A 2020 ACS Sustainale Chem. Eng. 8 9711
 Google Scholar
Google Scholar
[35] Gordillo-Vazquez F J 2008 J. Phys. D Appl. Phys. 41 234016
 Google Scholar
Google Scholar
[36] Kossyi I A, Kostinsky A Y, Matveyev A A, Silakov V P 1992 Plasma Sources Sci. Technol. 1 207
 Google Scholar
Google Scholar
计量
- 文章访问数: 5040
- PDF下载量: 124
- 被引次数: 0













 下载:
下载: