-
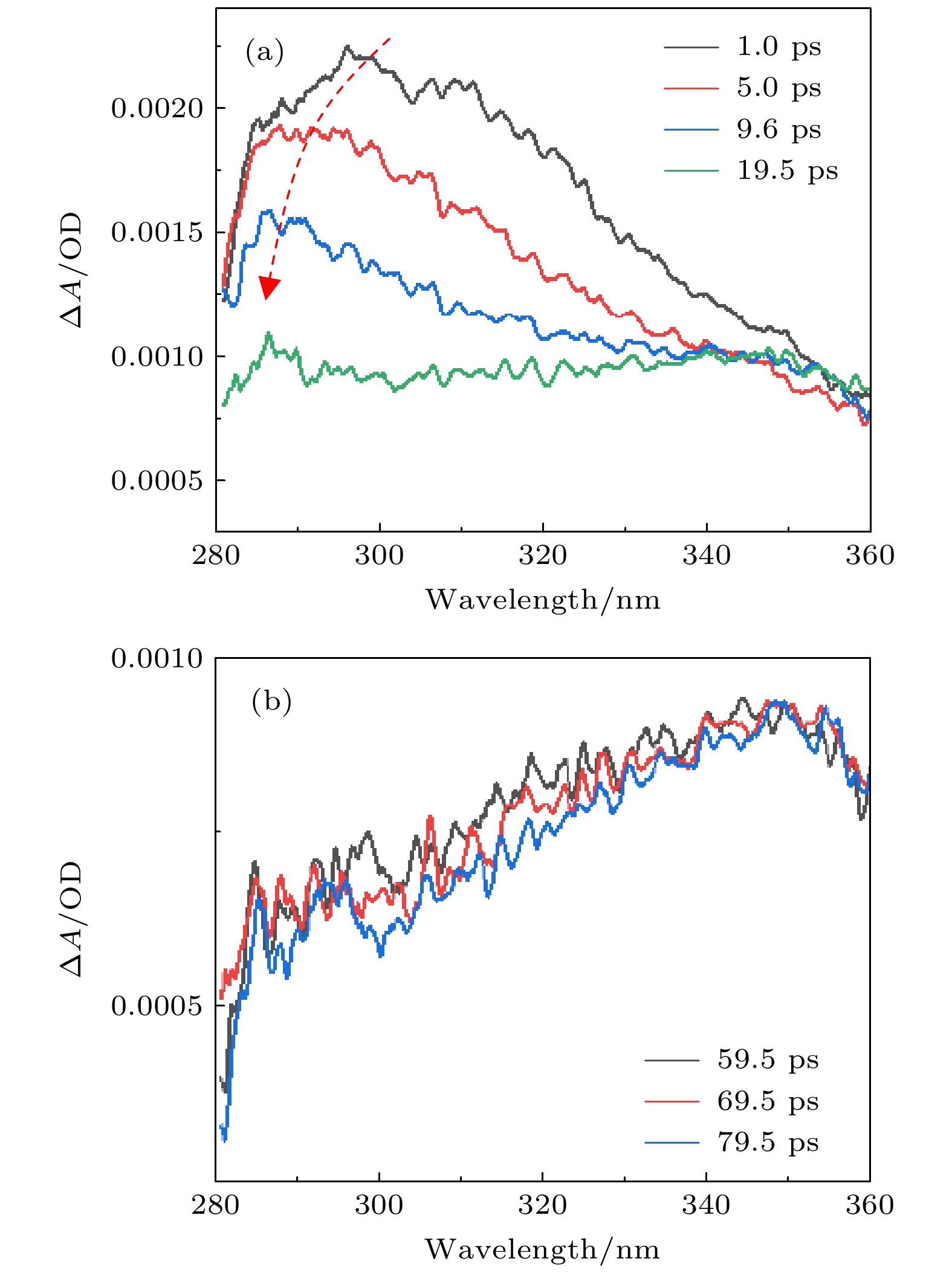
尿嘧啶是构成RNA链最基本的结构单元之一, 其光辐射下的光物理和光化学过程与光致癌变等过程紧密关联, 因而受到广泛的关注. 本文采用飞秒瞬态吸收光谱技术, 研究了在紫外光辐射下, 溶剂效应对尿嘧啶激发态超快动力学的影响. 实验利用一束264 nm的激发光将尿嘧啶激发到1(π, π*)态(即S2态). 然后, 利用覆盖范围为280—360 nm的紫外超连续谱作为探测光, 实现对尿嘧啶激发态和基态动力学的同步探测. 测量发现, 尿嘧啶在乙腈溶剂中显现了两个动力学衰减过程, 即9.8 ps (在300 nm处)和>1000 ps, 分别对应于振动冷却过程和三重态的衰减过程. 这一观察与水溶液中的结果差异很大, 因为在水溶液中基本没有测量到三重态的布居. 通过对一系列溶液展开测量, 发现尿嘧啶的超快动力学过程依赖于溶剂形成氢健能力的大小. 越容易形成氢键的溶剂, 尿嘧啶越不容易布居到三重态.As one of the building blocks in RNA chain, uracil and its derivatives have attracted a great deal of interest since its ultrafast dynamics is closely related to mutagenic and carcinogenic effects. In this study, the solvent effect on the ultrafast decay of excited uracil is studied by femtosecond transient absorption spectroscopy in the UV region. The uracil molecule is populated to the 1(π, π*) state (i.e. S2 state) with a pump pulse at 264 nm. Broad-band white light continuum in the UV region from 280 to 360 nm is used as the probe. With a detail analysis of the measured transient spectra, two decay time constants, i.e. 9.8 ps and > 1000 ps, are directly obtained at 300 nm in the solvent of acetonitrile. Compared with our previous experiments, where no obvious triplet population is observed in water, triplet population is found to play an important role in acetonitrile. A comparison of excited-state dynamics among different solvents is also carried out. It reveals that the decay from the 1(n, π*) state (i.e., S1 state) to the T1 state shows a clear dependence on the H bonding of the solvents. With stronger H bonding, the 1(n, π*) excited state decays faster and has less chance to transfer to the triplet state. These results suggest that only when the 1(n, π*) state has excess vibrational energy can it transit to the triplet state through the intersystem crossing process. With this new information obtained in the present measurement, the decay dynamics of uracil on the S2 excited state can be further understood.
-
Keywords:
- femtosecond transient absorption spectroscopy /
- uracil /
- excited-state dynamic /
- solvent effect
[1] Zierhut M, Roth W, Fischer I 2004 Phys. Chem. Chem. Phys. 6 5178
 Google Scholar
Google Scholar
[2] Pfeifer G P, You Y H, Besaratinia A 2005 Mutat. Res.- Fund. Mol. Mech. Mutagen 571 19
 Google Scholar
Google Scholar
[3] de Gruijl F R 1999 Eur. J. Cancer 35 2003
 Google Scholar
Google Scholar
[4] Gustavsson T, Sarkar N, Lazzarotto E, Markovitsi D, Improta R 2006 Chem. Phys. Lett 429 551
 Google Scholar
Google Scholar
[5] Hare P M, Crespo-Hernandez C E, Kohler B 2007 Proc. Natl. Acad. Sci. U. S. A 104 435
 Google Scholar
Google Scholar
[6] Gustavsson T, Banyasz A, Lazzarotto E, Markovitsi D, Scalmani G, Frisch M J, Barone V, Improta R 2006 J. Am. Chem. Soc 128 607
 Google Scholar
Google Scholar
[7] Matsika S 2004 J. Phys. Chem. A 108 7584
 Google Scholar
Google Scholar
[8] Barbatti M, Aquino A J A, Szymczak J J, Nachtigallova D, ́Hobza P, Lischka H 2010 Proc. Natl. Acad. Sci. U. S. A 107 21453
 Google Scholar
Google Scholar
[9] Hare P M, Crespo-Hernandez C E, Kohler B 2006 J. Phys. Chem. B 110 18641
 Google Scholar
Google Scholar
[10] Gustavsson T, Sarkar N, Lazzarotto E, Markovitsi D, Barone V, Improta R 2006 J. Phys. Chem. B 110 12843
 Google Scholar
Google Scholar
[11] Gustavsson T, Sarkar N, Banyasz A, Markovitsi D, Improta R 2007 Photochem. Photobiol 83 595
 Google Scholar
Google Scholar
[12] Hua X, Hua L, Liu X 2015 J. Phys. Chem. A 119 12985
 Google Scholar
Google Scholar
[13] Hua X, Hua L, Liu X 2016 Phys. Chem. Chem. Phys 18 13904
 Google Scholar
Google Scholar
[14] Li P, Xue J, Zheng X 2019 J. Raman. Spectrosc 50 345
 Google Scholar
Google Scholar
[15] Santoro F, Barone V, Gustavsson T, Improta R 2006 J. Am. Chem. Soc 128 16312
 Google Scholar
Google Scholar
[16] Improta R, Barone V 2008 Theor. Chem. Acc 120 491
 Google Scholar
Google Scholar
[17] Improta R, Barone V, Lami A, Santoro F 2009 J. Phys. Chem. B 113 14491
 Google Scholar
Google Scholar
[18] Etinski M, Marian C M 2010 Phys. Chem. Chem. Phys 12 15665
 Google Scholar
Google Scholar
[19] Danillo V, Adalberto V S A, Antonio C B 2021 Molecules 26 5191
 Google Scholar
Google Scholar
[20] Salet C, Bensasson R 1975 Photochem. Photobiol 22 231
 Google Scholar
Google Scholar
[21] Gorner H 1990 Photochem. Photobiol 52 935
 Google Scholar
Google Scholar
[22] Charvat A, Assmann J, Abel B, Schwarzer D, Henning K, Luther K, Troe J 2001 Phys. Chem. Chem. Phys 3 2230
 Google Scholar
Google Scholar
[23] Schwarzer D, Hanisch C, Kutne P, Troe J 2002 J. Phys. Chem. A 106 8019
 Google Scholar
Google Scholar
[24] Schwarzer D, Kutne P, Schroder C, Troe J 2004 J. Chem. Phys 121 1754
 Google Scholar
Google Scholar
[25] Schwarzer D, Troe J, Votsmeier M, Zerezke M 1996 J. Chem. Phys 105 3121
 Google Scholar
Google Scholar
[26] Schwarzer D, Troe J, Votsmeier M, Zerezke M 1997 Ber. Bunsen. Phys. Chem. 101 595
 Google Scholar
Google Scholar
-
表 1 在不同溶剂中观察到的时间常数
Table 1. Observed time constants in different solvents.
乙腈 乙酸乙酯 甲醇 乙二醇 水 a τ1/ps 9.8 6.8 3.5 3.1 1.1 b τ2/ps >1000 >1000 >1000 >1000 >1000 a 以300 nm处的衰减曲线为例; b τ2 在之前的研究中未能被精确测量. -
[1] Zierhut M, Roth W, Fischer I 2004 Phys. Chem. Chem. Phys. 6 5178
 Google Scholar
Google Scholar
[2] Pfeifer G P, You Y H, Besaratinia A 2005 Mutat. Res.- Fund. Mol. Mech. Mutagen 571 19
 Google Scholar
Google Scholar
[3] de Gruijl F R 1999 Eur. J. Cancer 35 2003
 Google Scholar
Google Scholar
[4] Gustavsson T, Sarkar N, Lazzarotto E, Markovitsi D, Improta R 2006 Chem. Phys. Lett 429 551
 Google Scholar
Google Scholar
[5] Hare P M, Crespo-Hernandez C E, Kohler B 2007 Proc. Natl. Acad. Sci. U. S. A 104 435
 Google Scholar
Google Scholar
[6] Gustavsson T, Banyasz A, Lazzarotto E, Markovitsi D, Scalmani G, Frisch M J, Barone V, Improta R 2006 J. Am. Chem. Soc 128 607
 Google Scholar
Google Scholar
[7] Matsika S 2004 J. Phys. Chem. A 108 7584
 Google Scholar
Google Scholar
[8] Barbatti M, Aquino A J A, Szymczak J J, Nachtigallova D, ́Hobza P, Lischka H 2010 Proc. Natl. Acad. Sci. U. S. A 107 21453
 Google Scholar
Google Scholar
[9] Hare P M, Crespo-Hernandez C E, Kohler B 2006 J. Phys. Chem. B 110 18641
 Google Scholar
Google Scholar
[10] Gustavsson T, Sarkar N, Lazzarotto E, Markovitsi D, Barone V, Improta R 2006 J. Phys. Chem. B 110 12843
 Google Scholar
Google Scholar
[11] Gustavsson T, Sarkar N, Banyasz A, Markovitsi D, Improta R 2007 Photochem. Photobiol 83 595
 Google Scholar
Google Scholar
[12] Hua X, Hua L, Liu X 2015 J. Phys. Chem. A 119 12985
 Google Scholar
Google Scholar
[13] Hua X, Hua L, Liu X 2016 Phys. Chem. Chem. Phys 18 13904
 Google Scholar
Google Scholar
[14] Li P, Xue J, Zheng X 2019 J. Raman. Spectrosc 50 345
 Google Scholar
Google Scholar
[15] Santoro F, Barone V, Gustavsson T, Improta R 2006 J. Am. Chem. Soc 128 16312
 Google Scholar
Google Scholar
[16] Improta R, Barone V 2008 Theor. Chem. Acc 120 491
 Google Scholar
Google Scholar
[17] Improta R, Barone V, Lami A, Santoro F 2009 J. Phys. Chem. B 113 14491
 Google Scholar
Google Scholar
[18] Etinski M, Marian C M 2010 Phys. Chem. Chem. Phys 12 15665
 Google Scholar
Google Scholar
[19] Danillo V, Adalberto V S A, Antonio C B 2021 Molecules 26 5191
 Google Scholar
Google Scholar
[20] Salet C, Bensasson R 1975 Photochem. Photobiol 22 231
 Google Scholar
Google Scholar
[21] Gorner H 1990 Photochem. Photobiol 52 935
 Google Scholar
Google Scholar
[22] Charvat A, Assmann J, Abel B, Schwarzer D, Henning K, Luther K, Troe J 2001 Phys. Chem. Chem. Phys 3 2230
 Google Scholar
Google Scholar
[23] Schwarzer D, Hanisch C, Kutne P, Troe J 2002 J. Phys. Chem. A 106 8019
 Google Scholar
Google Scholar
[24] Schwarzer D, Kutne P, Schroder C, Troe J 2004 J. Chem. Phys 121 1754
 Google Scholar
Google Scholar
[25] Schwarzer D, Troe J, Votsmeier M, Zerezke M 1996 J. Chem. Phys 105 3121
 Google Scholar
Google Scholar
[26] Schwarzer D, Troe J, Votsmeier M, Zerezke M 1997 Ber. Bunsen. Phys. Chem. 101 595
 Google Scholar
Google Scholar
计量
- 文章访问数: 5455
- PDF下载量: 103
- 被引次数: 0













 下载:
下载:



