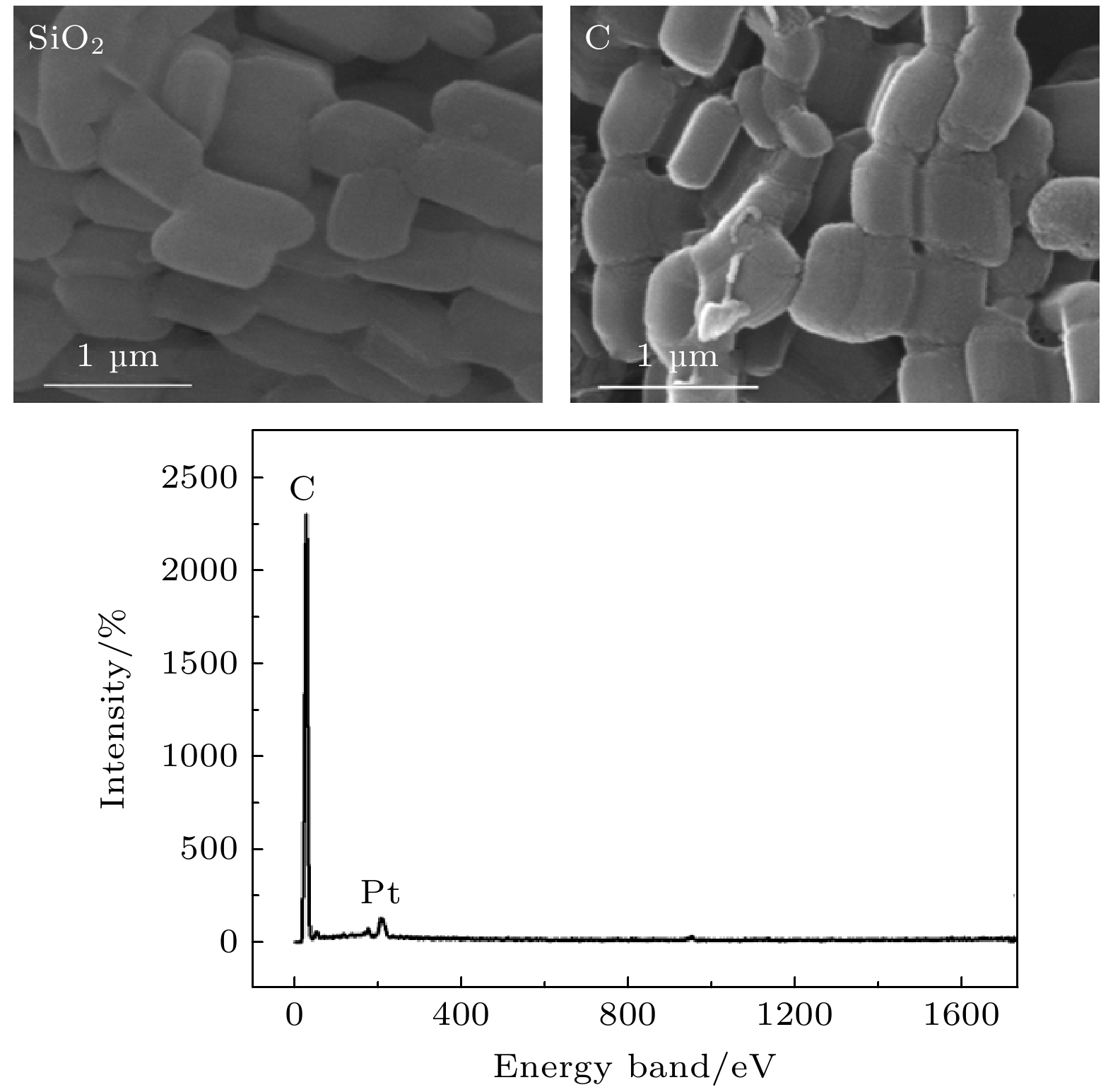
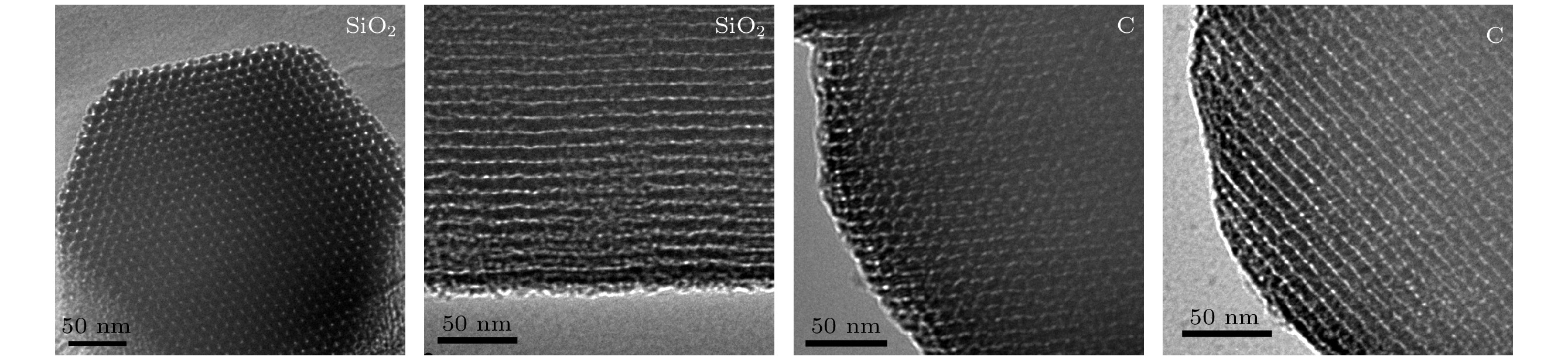
-
以P123为结构导向剂、TEOS为硅源制备了有序介孔二氧化硅SBA-15, 并以此为模板制备了有序介孔碳(OMC). 小角X射线衍射、高分辨透射电子显微镜和N2吸附/脱附等测试结果均证实SBA-15与OMC具有高度有序的孔结构、相对较高的比表面积, 且孔洞平均尺寸分别约为7.5 nm和3.3 nm. 分别采用固相反应法和浸渍填充法制备了OMC/SBA-15复合材料和OMC@SBA-15及CuO@SBA-15复合材料. 随着OMC和CuO质量分数的增大, 3种复合材料中o-Ps寿命
$ {\tau }_{4} $ 和其强度$ {I}_{4} $ 均减小. o-Ps湮没率$ {\lambda }_{4} $ 随OMC和CuO质量分数的变化可用一条或两条直线很好地拟合, OMC/SBA-15, OMC@SBA-15及CuO@SBA-15复合材料中反应速率常数$ k $ 分别为$(2.39\pm 0.44)\times {10}^{7}~{\mathrm{s}}^{-1}$ /$(6.65\pm 0.94)\times {10}^{6}~{\mathrm{s}}^{-1}$ ,$(2.28\pm 0.19)\times {10}^{7}~{\mathrm{s}}^{-1}$ 和$(8.76\pm 0.47)\times {10}^{6}~{\mathrm{s}}^{-1}$ . 因此, 3种复合材料中$ {\tau }_{4} $ 及$ {I}_{4} $ 降低是由于电子偶素与碳、铜元素在介孔内或孔表面发生了化学猝灭和禁止效应. 同时, 电子偶素也是一种检测多孔材料中孔隙结构的有效探针.Owing to highly ordered two-dimensional hexagonal structure, large surface area, variable pore size, high thermal stability and especially the electron delocalization energy determined by its frame structure, SBA-15 catalysts have received more and more researchers’ attention. By using the structure-directing agent of P123 and the silicon source of TEOS, we synthesize ordered mesoporous silica SBA-15. At the same time, ordered mesoporous carbon OMC is succefully synthesized with the template of SBA-15. The small angle X-ray diffraction, high resolution transmission electron microscopy and N2 adsorption-desorption measurements are conducted to verify the highly ordered pore structure and relatively high specific surface area of SBA-15 and OMC, and their average pore radius are about 7.5 nm and 3.3 nm, respectively. Positron lifetime spectrum of SBA-15 catalyst is composed of two longer lifetimes and two shorter lifetimes: two longer lifetimes$ {\tau }_{3} $ and$ {\tau }_{4} $ are the annihilation in micropore and large pore of positronium (Ps), are 7.5 ns and 106 ns. However, there is nearly no longer lifetime component in OMC, which indicates that there might exist the quenching or inhibiting of positronium by carbon material. To verify this guess, we synthesize the catalysts of OMC/SBA-15, OMC@SBA-15 and CuO@SBA-15 by the solid state reaction and the impregnation filling method. With the increasing of OMC and CuO content, both the o-Ps lifetime$ {\tau }_{4} $ and its intensity$ {I}_{4} $ of these three compounds decrease. The annihilation rate of o-Ps lifetime varying with OMC and CuO content can be better fitted by one or two straight lines, The values of reaction rate constant K in OMC/SBA-15, OMC@SBA-15 and CuO@SBA-15 are$(2.39\pm $ $ 0.44)\times {10}^{7}~{\mathrm{s}}^{-1}$ /$(6.65\pm 0.94)\times {10}^{6}~{\mathrm{s}}^{-1}$ ,$(2.28\pm 0.19)\times {10}^{7}~{\mathrm{s}}^{-1}$ , and$(8.76\pm 0.47)\times {10}^{6}~{\mathrm{s}}^{-1},$ respectively. Therefore, our results indicate that there are quenching effect and inhibition effect among the carbon, the CuO and the positronium, which lead$ {\tau }_{4} $ and$ {I}_{4} $ to decrease, and positronium is also a probe for detecting the pore structure of porous material.-
Keywords:
- positron annihilation spectrum /
- ordered mesoporous material /
- positronium /
- chemical quenching
[1] Veisi H, Ozturk T, Karmakar B, Tamoradi T, Hemmati S 2020 Carbohydr. Polym. 235 115966
 Google Scholar
Google Scholar
[2] Veisi H, Tamoradi T, Karmakar B, Hemmati S 2020 J. Phys. Chem. Solids 138 109256
 Google Scholar
Google Scholar
[3] Tamoradi T, Daraie M, Heravi M M, Karmakar B 2020 New J. Chem. 44 11049
 Google Scholar
Google Scholar
[4] Tamoradi T, Daraie M, Heravi M M 2020 Appl. Organomet Chem. 34 5538
 Google Scholar
Google Scholar
[5] Rehman F, Volpe P L O, Airoldi C 2014 J. Environ. Manage. 133 135
 Google Scholar
Google Scholar
[6] Xu Y, Hu E, Xu D, Guo Q 2021 Sep. Purif. Technol. 274 119081
 Google Scholar
Google Scholar
[7] Cao T, Wang C, Zhou Z, Liu L, Xu S, Song H, Lin W, Xu Z 2021 Appl. Surf. Sci. 552 149487
 Google Scholar
Google Scholar
[8] El-Denglawey A, Mubarak M F, Selim H 2021 Arab. J. Sci. Eng. 47 455
 Google Scholar
Google Scholar
[9] Yu N Y, Wu K, Tao L 2021 Chemosphere 276 130112
 Google Scholar
Google Scholar
[10] Kumaravel S, Thiripuranthagan S, Vembuli T, Erusappan E, Durai M, Sureshkumar T, Durai M 2021 Optik 235 166599
 Google Scholar
Google Scholar
[11] Chang Q, Yang S, Xue C, Li N, Wang Y, Li Y, Wang H, Yang J, Hu S 2019 Nanoscale 11 7247
 Google Scholar
Google Scholar
[12] Yang H C, Lin H Y, Chien Y S, Wu J C S, Wu H H 2009 Catal. Lett. 131 381
 Google Scholar
Google Scholar
[13] He J H, Xie T P, Luo T H, Xu Q, Ye F, An J B, Yang J, Wang J K 2021 Ecotox. Environ. Safe. 216 112189
 Google Scholar
Google Scholar
[14] Poonia E, Duhan S, Kumar K, Kumar A, Jakhar S, Tomer V K 2018 J. Porous Ma. 26 553
 Google Scholar
Google Scholar
[15] Sharma S K, Sudarshan K, Sen D, Pujari P K 2019 J. Solid State Chem. 274 10
 Google Scholar
Google Scholar
[16] Jean Y C, Mallon P E, Schrader D M 2003 Principles and Applications of Positron & Positronium Chemistry (Singapore: World Scientific Publishing)
[17] Sing K S, Everett D H, Haul R A W, Moscou L, Pierotti R A, Rouquerol J 1985 Pure Appl. Chem. 57 603
 Google Scholar
Google Scholar
[18] Tao S J 1972 J. Chem. Phys. 56 5499
 Google Scholar
Google Scholar
[19] Eldrup M, Lightbody D, Sherwood J N 1981 Chem. Phys. 63 51
 Google Scholar
Google Scholar
[20] Hyodo T, Nakayama T, Saito H, Saito F, Wada K 2009 Phys. Status Solidi (c) 6 2497
 Google Scholar
Google Scholar
[21] Varisov A Z, Grafutin V I, Zaluzhnyi A G, Ilyukhina O V, Myasishcheva G G, Prokop'ev E P, Timoshenkov S P, Funtikov Y V 2008 J. Surf. Ingestig. 2 959
 Google Scholar
Google Scholar
[22] Kim T W, Ryoo R, Gierszal K P, Jaroniec M, Solovyov L A, Sakamoto Y, Terasaki O 2005 J. Mater. Chem. 15 1560
 Google Scholar
Google Scholar
[23] Zhang H J, Chen Z Q, Wang S J, Kawasuso A, Morishita N 2010 Phys. Rev. B 82 035439
 Google Scholar
Google Scholar
[24] Sagara A, Yabe H, Chen X, Vereecken P M, Uedono A 2020 Microporous Mesoporous Mater. 295 109964
 Google Scholar
Google Scholar
[25] Zhao D Y, Feng J L, Huo Q S, Melosh N, Fredrickson G H, Chmelka B F, Stucky Galen D 1998 Science 279 548
 Google Scholar
Google Scholar
[26] Jun S, Joo S H, Ryoo R, Kruk M, Jaroniec M, Liu Z, Ohsuna T, Terasaki O 2000 J. Am. Chem. Soc. 122 10712
 Google Scholar
Google Scholar
[27] Brunauer S, Emmett P H, Teller E 1938 J. Am. Chem. Soc. 60 309
 Google Scholar
Google Scholar
[28] Barrett E P, Joyner L G, Halenda P P 1951 J. Am. Chem. Soc. 73 373
 Google Scholar
Google Scholar
[29] Davis M E 2002 Nature 417 813
 Google Scholar
Google Scholar
[30] Paulin P R, Ambrosino G 1968 J. Phys. France 29 263
 Google Scholar
Google Scholar
[31] Dull T L, Frieze W E, Gidley D W, 2001 J. Phys. Chem. B 105 4657
 Google Scholar
Google Scholar
[32] Goworek T, Jasinska B, Wawryszczuk J 1998 Chem. Phys. 230 305
 Google Scholar
Google Scholar
[33] Zhang H J, Chen Z Q, Wang S J 2012 J. Chem. Phys. 136 034701
 Google Scholar
Google Scholar
[34] Saito H, Hyodo T 1999 Phys. Rev. B 60 11070
 Google Scholar
Google Scholar
[35] Li C Y, Zhao B, Zhou B, Qi N, Chen Z Q, Zhou W 2017 Phys. Chem. Chem. Phys. 19 7659
 Google Scholar
Google Scholar
[36] Sudarshan K, Patil P N, Goswami A, Pillai K T, Pujari P K 2009 Phys. Status Solidi (c) 6 2552
 Google Scholar
Google Scholar
-
图 4 有序介孔碳、其模板二氧化硅及CuO质量分数分别为1%, 1.5%, 2%的CuO@SBA-15复合材料的N2吸附/脱附等温线及相应的孔径分布(STP代表标准状况)
Fig. 4. N2 adsorption and desorption measurement of synthesized ordered mesoporous carbon, its template silica and CuO@SBA-15 composite materials with the CuO weight content of 1%, 1.5%, 2% (STP, standard temperature and pressure)
图 9 不同OMC质量分数的OMC/ SBA-15, OMC@SBA-15复合材料中o-Ps寿命
$ {\tau }_{3} $ ,$ {\tau }_{4} $ ,${\tau }_{3}'$ ,${\tau }_{4}'$ 的变化, 其中$ {\tau }_{3} $ ,$ {\tau }_{4} $ 为浸渍填充法制备OMC@SBA-15复合材料的测试结果,${\tau }_{3}'$ ,${\tau }_{4}'$ 为固相混合法制备OMC/SBA-15复合材料的测试结果Fig. 9. Variation of
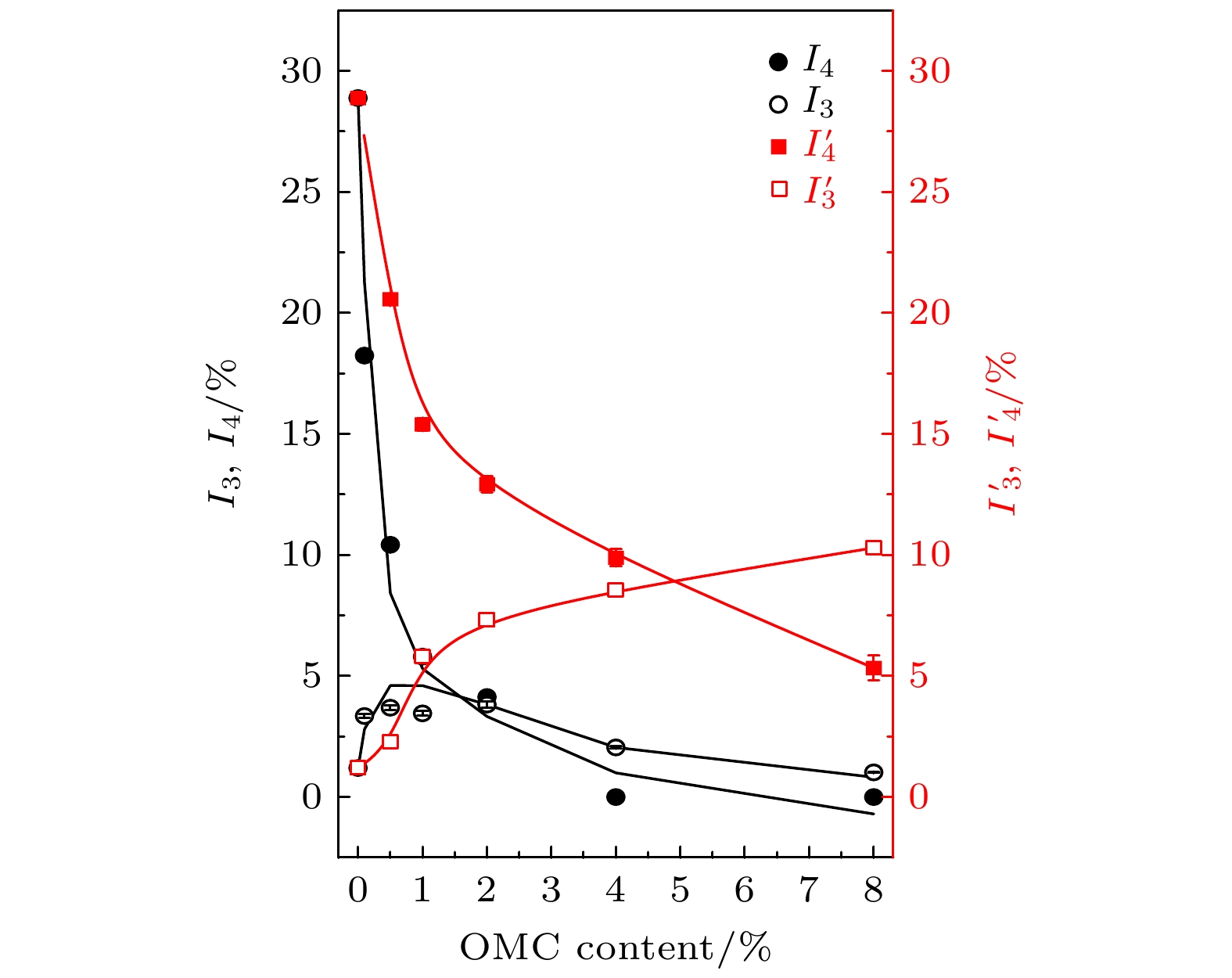
$ {\tau }_{3} $ ,$ {\tau }_{4} $ ,${\tau }_{3}'$ ,${\tau }_{4}'$ parameter with the weight content of OMC in OMC/SBA-15 and OMC@SBA-15 components.$ {\tau }_{3} $ ,$ {\tau }_{4} $ for the results of OMC@SBA-15 component synthesized by impregnation method,${\tau }_{3}'$ ,${\tau }_{4}'$ for that of OMC/SBA-15 component synthesized by solid state method.图 10 不同OMC质量分数的OMC/SBA-15, OMC@SBA-15复合材料中o-Ps寿命强度
$ {I}_{3} $ ,$ {I}_{4} $ ,${I}_{3}'$ ,${I}_{4}'$ 的变化, 其中$ {I}_{3} $ ,$ {I}_{4} $ 为浸渍填充法制备OMC@SBA-15复合材料的测试结果,${I}_{3}'$ ,${I}_{4}'$ 为固相混合法制备OMC/SBA-15复合材料的测试结果Fig. 10. Variation of the intensity of o-Ps lifetime
$ {I}_{3} $ ,$ {I}_{4} $ ,${I}_{3}'$ ,${I}_{4}'$ parameter with the weight content of OMC in OMC/SBA-15 and OMC@SBA-15 components.$ {I}_{3} $ ,$ {I}_{4} $ for the results of OMC@SBA-15 component synthesized by impregnation method,${I}_{3}'$ ,${I}_{4}'$ for that of OMC/SBA-15 component synthesized by solid state method.图 11 不同OMC质量分数的OMC/SBA-15, OMC@SBA-15复合材料中
$ {\lambda }_{4} $ ($ 1/{\tau }_{4} $ ),${\lambda }_{4}'\,(1/{\tau }_{4}')$ 的变化, 其中$ {\lambda }_{4} $ ($ 1/{\tau }_{4} $ )为浸渍填充法制备OMC@SBA-15复合材料的测试结果, 而${{\lambda }_{4}'\, (1/{\tau }_{4}')}$ 为固相混合法制备OMC/SBA-15复合材料的测试结果Fig. 11. Variation of the intensity of o-Ps lifetime
${\lambda }_{4}\,(1/{\tau }_{4})$ ,${\lambda }_{4}'\, (1/{\tau }_{4}')$ parameter with the weight content of OMC in OMC/SBA-15 and OMC@SBA-15 components.${\lambda }_{4}\,(1/{\tau }_{4})$ or the results of OMC@SBA-15 component synthesized by impregnation method,${\lambda }_{4}'\, (1/{\tau }_{4}')$ for that of OMC/SBA-15 component synthesized by solid state method.图 12 不同OMC质量分数的OMC/SBA-15, OMC@SBA-15复合材料中
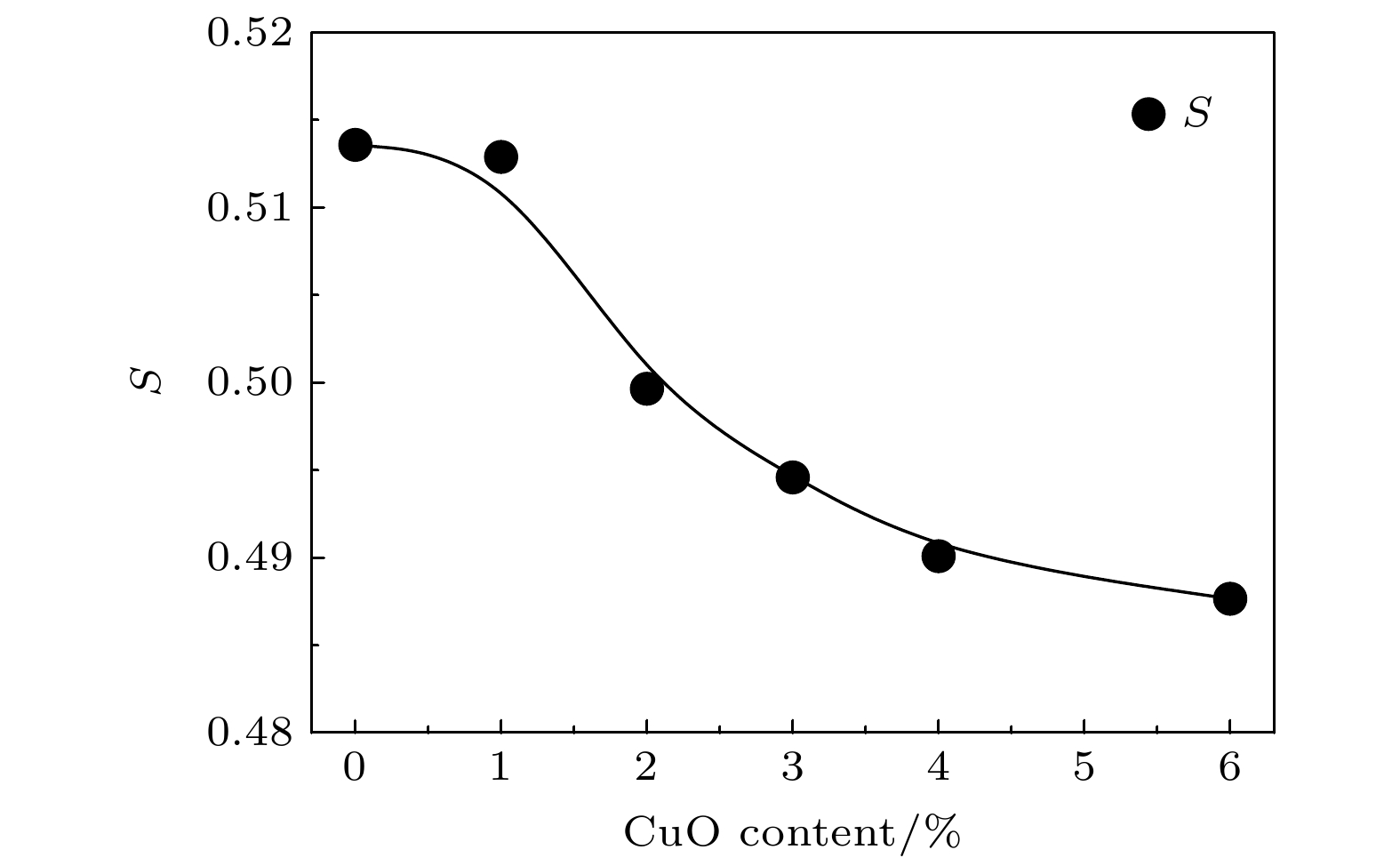
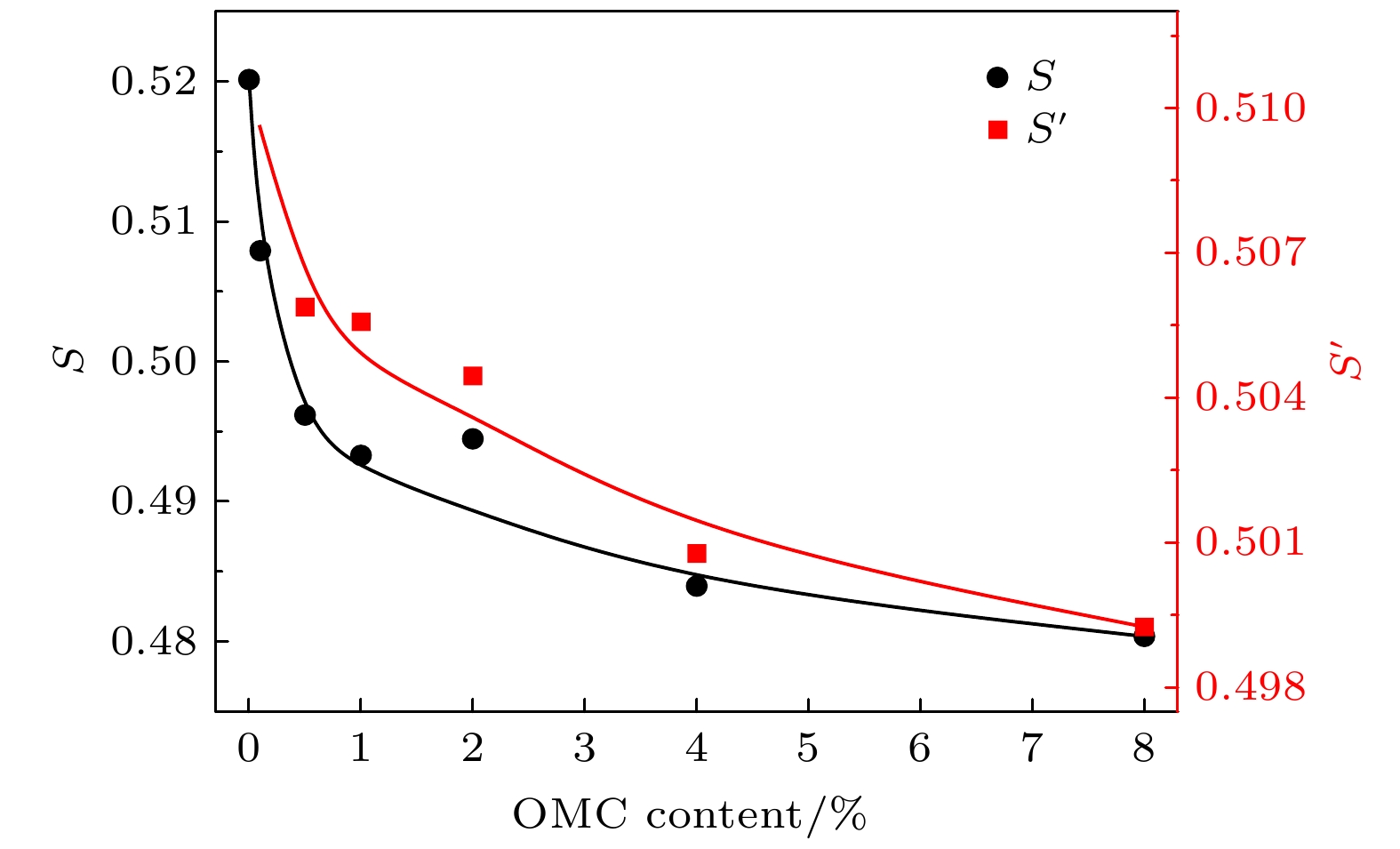
$ S $ 和${S}{'}$ 参数的变化, 其中$ S $ 为浸渍填充法制备OMC@SBA-15复合材料的测试结果, 而${S}{'}$ 为固相混合法制备OMC/SBA-15复合材料的测试结果Fig. 12. Variation of the intensity of o-Ps lifetime
$ S $ ,${S}{'}$ parameter with the weight content of OMC in OMC/SBA-15 and OMC@SBA-15 components.$ S $ or the results of OMC@SBA-15 synthesized by impregnation method,${S}{'}$ for that of OMC/SBA-15 synthesized by solid state method. -
[1] Veisi H, Ozturk T, Karmakar B, Tamoradi T, Hemmati S 2020 Carbohydr. Polym. 235 115966
 Google Scholar
Google Scholar
[2] Veisi H, Tamoradi T, Karmakar B, Hemmati S 2020 J. Phys. Chem. Solids 138 109256
 Google Scholar
Google Scholar
[3] Tamoradi T, Daraie M, Heravi M M, Karmakar B 2020 New J. Chem. 44 11049
 Google Scholar
Google Scholar
[4] Tamoradi T, Daraie M, Heravi M M 2020 Appl. Organomet Chem. 34 5538
 Google Scholar
Google Scholar
[5] Rehman F, Volpe P L O, Airoldi C 2014 J. Environ. Manage. 133 135
 Google Scholar
Google Scholar
[6] Xu Y, Hu E, Xu D, Guo Q 2021 Sep. Purif. Technol. 274 119081
 Google Scholar
Google Scholar
[7] Cao T, Wang C, Zhou Z, Liu L, Xu S, Song H, Lin W, Xu Z 2021 Appl. Surf. Sci. 552 149487
 Google Scholar
Google Scholar
[8] El-Denglawey A, Mubarak M F, Selim H 2021 Arab. J. Sci. Eng. 47 455
 Google Scholar
Google Scholar
[9] Yu N Y, Wu K, Tao L 2021 Chemosphere 276 130112
 Google Scholar
Google Scholar
[10] Kumaravel S, Thiripuranthagan S, Vembuli T, Erusappan E, Durai M, Sureshkumar T, Durai M 2021 Optik 235 166599
 Google Scholar
Google Scholar
[11] Chang Q, Yang S, Xue C, Li N, Wang Y, Li Y, Wang H, Yang J, Hu S 2019 Nanoscale 11 7247
 Google Scholar
Google Scholar
[12] Yang H C, Lin H Y, Chien Y S, Wu J C S, Wu H H 2009 Catal. Lett. 131 381
 Google Scholar
Google Scholar
[13] He J H, Xie T P, Luo T H, Xu Q, Ye F, An J B, Yang J, Wang J K 2021 Ecotox. Environ. Safe. 216 112189
 Google Scholar
Google Scholar
[14] Poonia E, Duhan S, Kumar K, Kumar A, Jakhar S, Tomer V K 2018 J. Porous Ma. 26 553
 Google Scholar
Google Scholar
[15] Sharma S K, Sudarshan K, Sen D, Pujari P K 2019 J. Solid State Chem. 274 10
 Google Scholar
Google Scholar
[16] Jean Y C, Mallon P E, Schrader D M 2003 Principles and Applications of Positron & Positronium Chemistry (Singapore: World Scientific Publishing)
[17] Sing K S, Everett D H, Haul R A W, Moscou L, Pierotti R A, Rouquerol J 1985 Pure Appl. Chem. 57 603
 Google Scholar
Google Scholar
[18] Tao S J 1972 J. Chem. Phys. 56 5499
 Google Scholar
Google Scholar
[19] Eldrup M, Lightbody D, Sherwood J N 1981 Chem. Phys. 63 51
 Google Scholar
Google Scholar
[20] Hyodo T, Nakayama T, Saito H, Saito F, Wada K 2009 Phys. Status Solidi (c) 6 2497
 Google Scholar
Google Scholar
[21] Varisov A Z, Grafutin V I, Zaluzhnyi A G, Ilyukhina O V, Myasishcheva G G, Prokop'ev E P, Timoshenkov S P, Funtikov Y V 2008 J. Surf. Ingestig. 2 959
 Google Scholar
Google Scholar
[22] Kim T W, Ryoo R, Gierszal K P, Jaroniec M, Solovyov L A, Sakamoto Y, Terasaki O 2005 J. Mater. Chem. 15 1560
 Google Scholar
Google Scholar
[23] Zhang H J, Chen Z Q, Wang S J, Kawasuso A, Morishita N 2010 Phys. Rev. B 82 035439
 Google Scholar
Google Scholar
[24] Sagara A, Yabe H, Chen X, Vereecken P M, Uedono A 2020 Microporous Mesoporous Mater. 295 109964
 Google Scholar
Google Scholar
[25] Zhao D Y, Feng J L, Huo Q S, Melosh N, Fredrickson G H, Chmelka B F, Stucky Galen D 1998 Science 279 548
 Google Scholar
Google Scholar
[26] Jun S, Joo S H, Ryoo R, Kruk M, Jaroniec M, Liu Z, Ohsuna T, Terasaki O 2000 J. Am. Chem. Soc. 122 10712
 Google Scholar
Google Scholar
[27] Brunauer S, Emmett P H, Teller E 1938 J. Am. Chem. Soc. 60 309
 Google Scholar
Google Scholar
[28] Barrett E P, Joyner L G, Halenda P P 1951 J. Am. Chem. Soc. 73 373
 Google Scholar
Google Scholar
[29] Davis M E 2002 Nature 417 813
 Google Scholar
Google Scholar
[30] Paulin P R, Ambrosino G 1968 J. Phys. France 29 263
 Google Scholar
Google Scholar
[31] Dull T L, Frieze W E, Gidley D W, 2001 J. Phys. Chem. B 105 4657
 Google Scholar
Google Scholar
[32] Goworek T, Jasinska B, Wawryszczuk J 1998 Chem. Phys. 230 305
 Google Scholar
Google Scholar
[33] Zhang H J, Chen Z Q, Wang S J 2012 J. Chem. Phys. 136 034701
 Google Scholar
Google Scholar
[34] Saito H, Hyodo T 1999 Phys. Rev. B 60 11070
 Google Scholar
Google Scholar
[35] Li C Y, Zhao B, Zhou B, Qi N, Chen Z Q, Zhou W 2017 Phys. Chem. Chem. Phys. 19 7659
 Google Scholar
Google Scholar
[36] Sudarshan K, Patil P N, Goswami A, Pillai K T, Pujari P K 2009 Phys. Status Solidi (c) 6 2552
 Google Scholar
Google Scholar
计量
- 文章访问数: 5817
- PDF下载量: 64
- 被引次数: 0





























 下载:
下载: