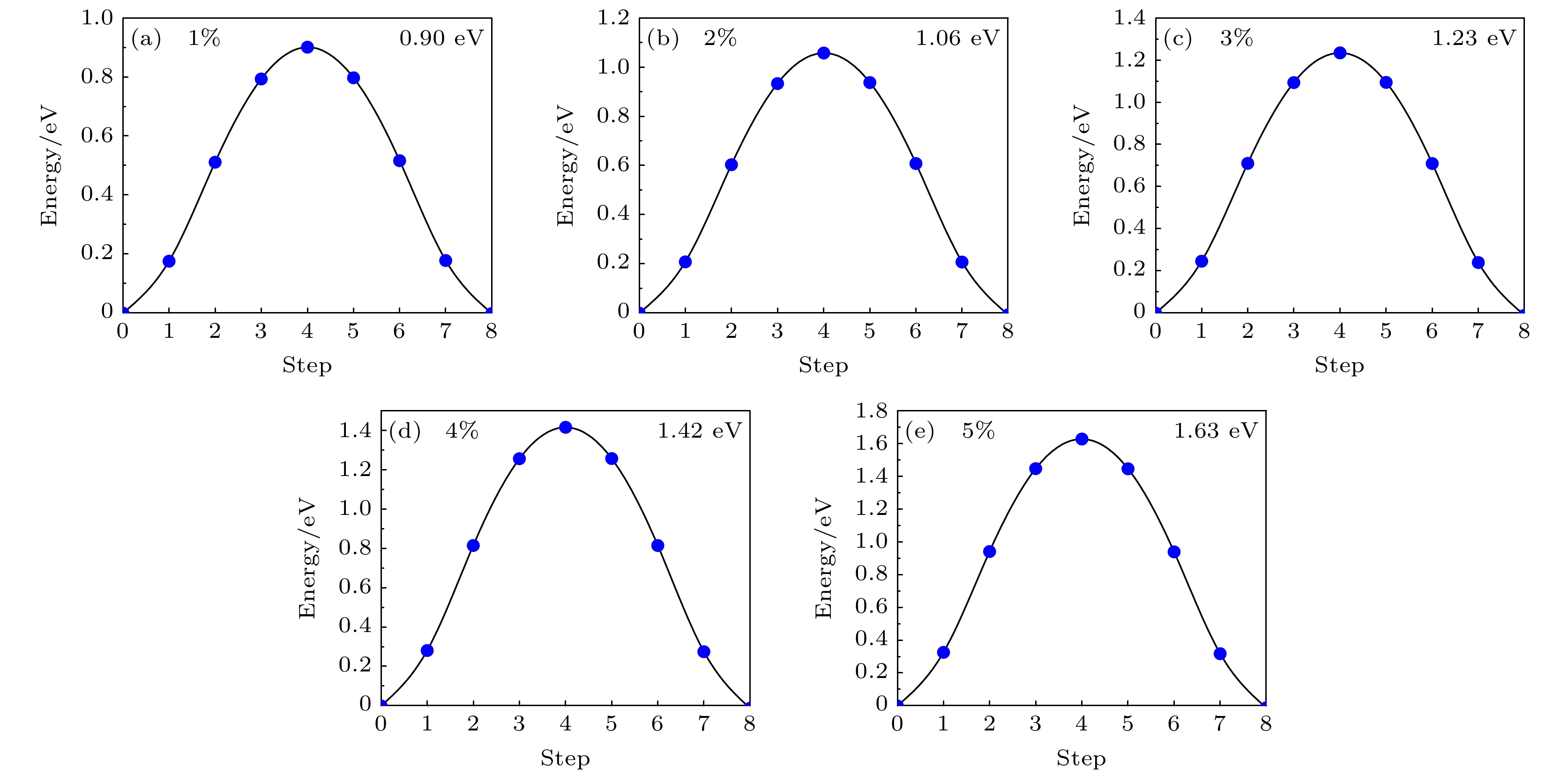
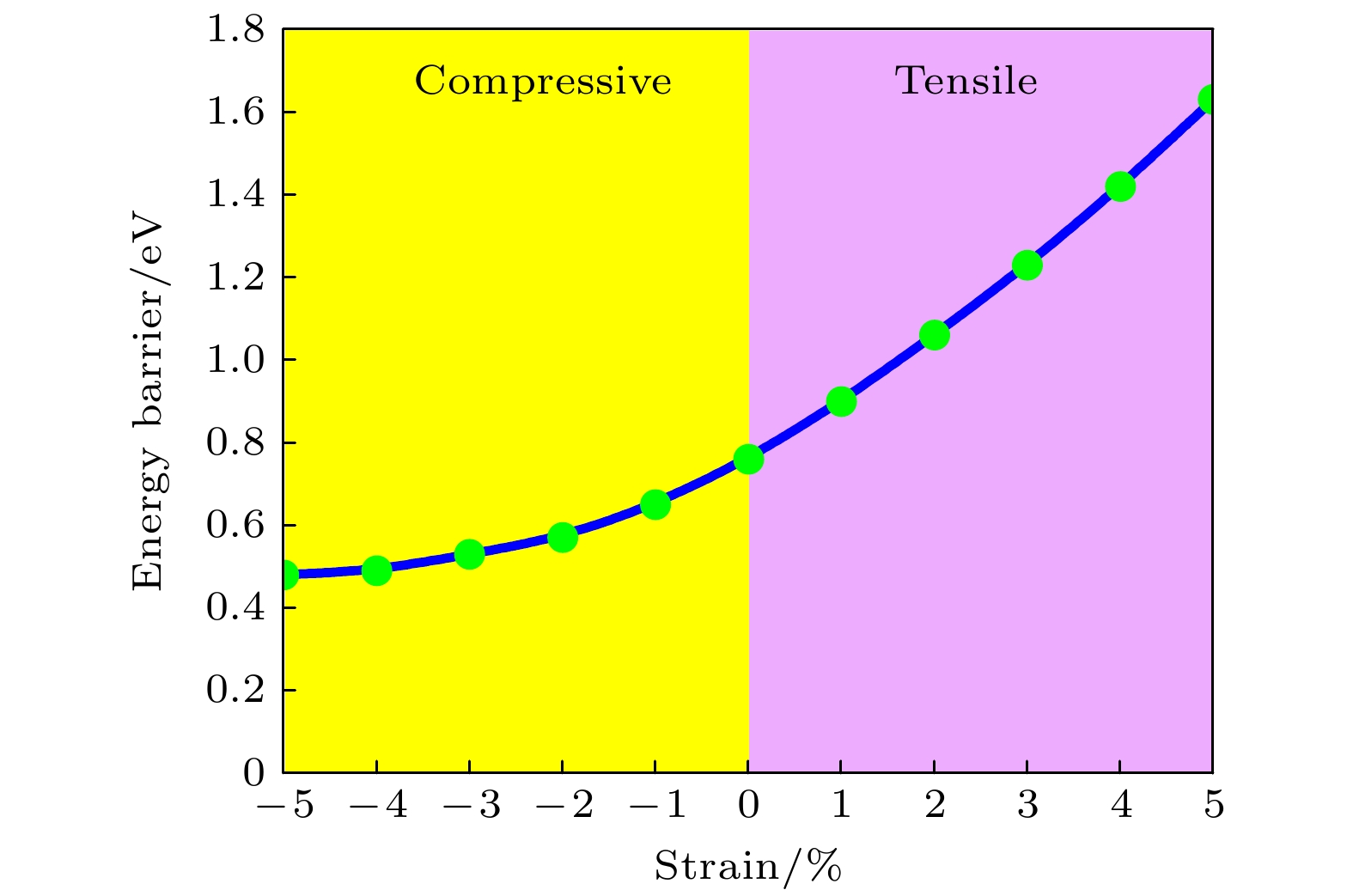
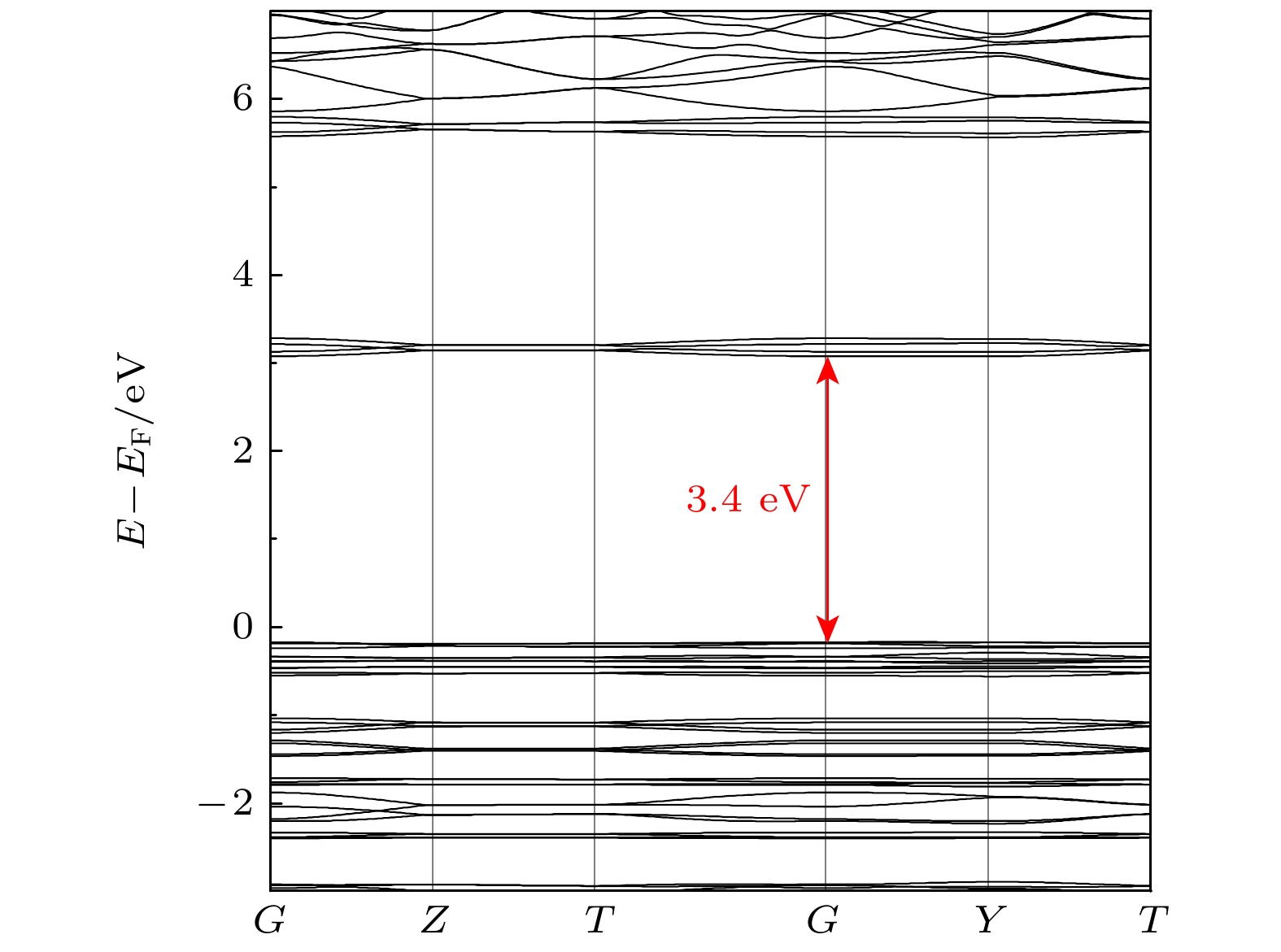
-
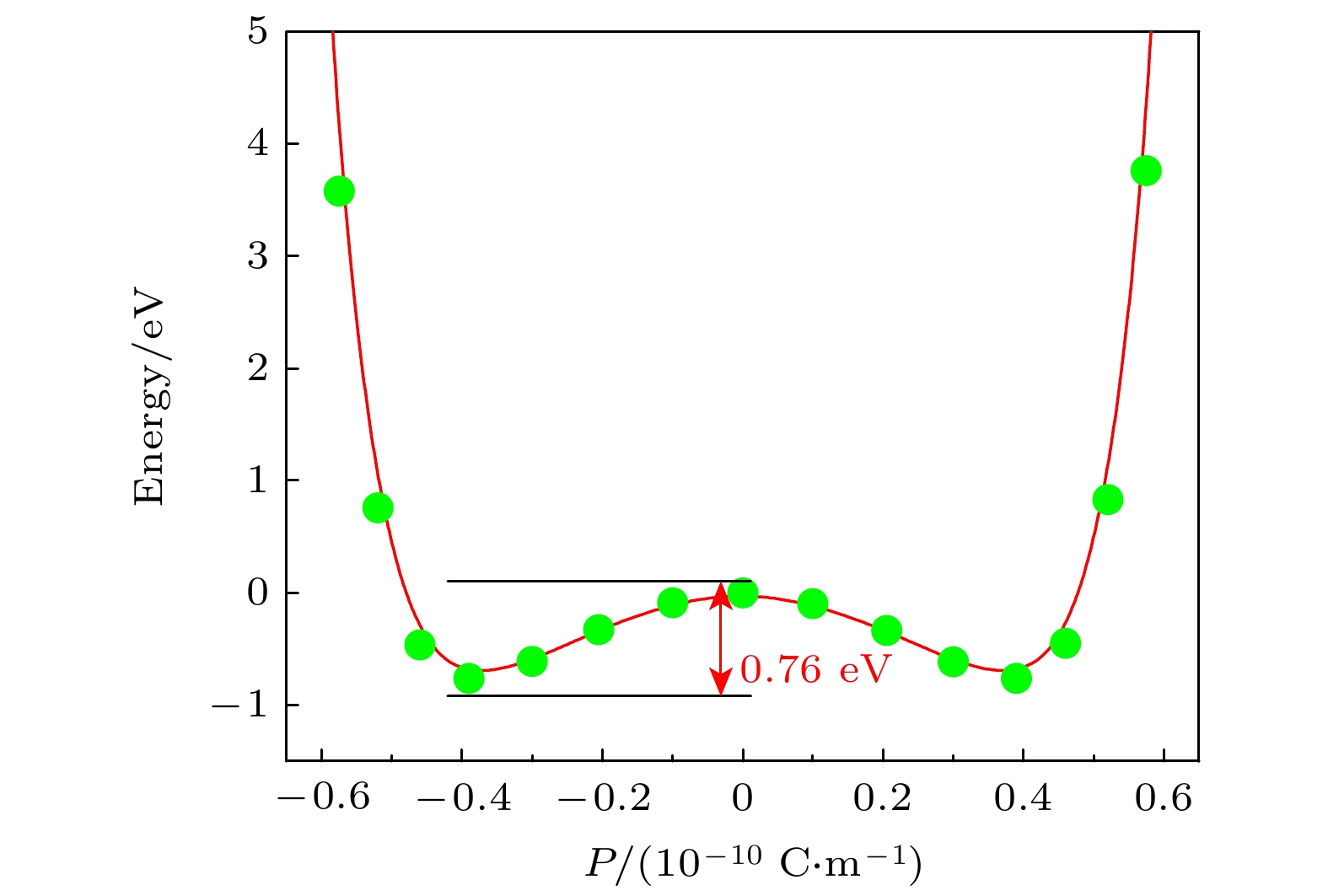
有机分子铁电材料相较于传统无机铁电材料具有轻质、柔性、不含重金属原子和成本低等诸多优点, 长期以来得到了广泛的关注和研究. 近年来, 原子厚度的二维无机铁电材料的研究取得了突破性进展, 因而备受关注, 然而二维有机铁电材料的设计与研究却鲜有报道. 本文基于密度泛函理论方法设计了一种以环丁烯-1,2-二羧酸(cyclobutene-1,2-dicarboxylic acid, CBDC)分子为结构单元的二维单层有机铁电分子晶体. 由于CBDC分子晶体内部氢键的链状排布, 导致其块体呈现出明显的层状结构, 计算发现内部的氢键链使得CBDC分子晶体块体具有各向异性的剥离能, 因此有望由沿着剥离能最低的(102)晶面进行机械/化学剥离而获得相应的单层有机铁电分子晶体. 理论计算预测CBDC (102)分子晶体单层的面内自发极化约0.39 × 10–6 μC/cm, 可与部分无机同类相比拟. 计算表明CBDC (102)分子晶体单层具有较高的极化反转势垒, 且对外加单轴应力的响应较为敏感. CBDC (102)单层有机铁电分子晶体的高面内自发极化以及易被界面调控的极化反转势垒使其可被应用于轻质无金属及柔性铁电器件.Compared with traditional inorganic ferroelectric materials, organic molecular ferroelectric materials possess many advantages, such as light weight, flexibility, no heavy metal atoms and low cost, and have received extensive attention for a long time. In recent years, atomic-thick two-dimensional (2D) inorganic ferroelectric materials have achieved breakthrough and attracted much attention. However, there are few reports on the design and research of two-dimensional organic ferroelectric materials. In this paper, we theoretically propose a 2D monolayer organic ferroelectric molecular crystal with the cyclobutene-1,2-dicarboxylic acid (CBDC) molecules as the building block based on density functional theory calculations. The bulk of CBDC molecular crystals clearly shows layered structure due to the chain-like arrangement of hydrogen bonds in crystal. It is found that the internal hydrogen bond chains give rise to the anisotropic cleavage energy values along different crystal planes of the CBDC molecular crystal bulk. Theoretical calculation suggests that the CBDC based 2D monolayer organic ferroelectric molecular crystal can be achieved by the mechanical/chemical peeling along the (102) crystal plane because of the lowest cleavage energy. It is predicted that the in-plane spontaneous polarization of the CBDC (102) molecular crystal monolayer is ~0.39 × 10–6 μC/cm, which is comparable to those of some inorganic counterparts. Calculations also indicate that the CBDC (102) molecular crystal monolayer shows a high polarization reversal barrier and is sensitive to the external uniaxial stress. The CBDC (102) monolayer organic ferroelectric molecular crystal reveals high in-plane spontaneous polarization with polarization reversal barrier easily modulated by the interface strain engineering, thereby rendering it great potential in lightweight, metal-free and flexible ferroelectric devices.
-
Keywords:
- two-dimensional molecular crystal /
- organic ferroelectric /
- in-plane polarization /
- polarization reversal
[1] Novoselov K S, Geim A K, Morozov S V, Jiang D, Zhang Y, Dubonos S V, Grigorieva I V, Firsov A A 2004 Science 306 666
 Google Scholar
Google Scholar
[2] Radisavljevic B, Radenovic A, Brivio J, Giacometti V, Kis A 2011 Nat. Nanotechnol. 6 147
 Google Scholar
Google Scholar
[3] Lopez-Sanchez O, Lembke D, Kayci M, Radenovic A, Kis A 2013 Nat. Nanotechnol. 8 497
 Google Scholar
Google Scholar
[4] Zeng H, Dai J, Yao W, Xiao D, Cui X 2012 Nat. Nanotechnol. 7 490
 Google Scholar
Google Scholar
[5] Cong C, Shang J, Wu X, Cao B, Peimyoo N, Qiu C, Sun L, Yu T 2014 Adv. Opt. Mater. 2 131
 Google Scholar
Google Scholar
[6] Bertolazzi S, Brivio J, Kis A 2011 ACS Nano 5 9703
 Google Scholar
Google Scholar
[7] Cai Y, Lan J, Zhang G, Zhang Y W 2014 Phys. Rev. B 89 035438
 Google Scholar
Google Scholar
[8] Profeta G, Calandra M, Mauri F 2012 Nat. Phys. 8 131
 Google Scholar
Google Scholar
[9] Zhou J J, Feng W, Liu C C, Guan S, Yao Y 2014 Nano Lett. 14 4767
 Google Scholar
Google Scholar
[10] Li X, Yang J 2014 J. Mater. Chem. C 8 8098
 Google Scholar
Google Scholar
[11] Belianinov A, He Q, Dziaugys A, Maksymovych P, Eliseev E, Borisevich A, Morozovska A, Banys J, Vysochanskii Y, Kalinin S V 2015 Nano Lett. 15 3808
 Google Scholar
Google Scholar
[12] Bune A V, Fridkin V M, Ducharme S, Blinov L M, Palto S P, Sorokin A V, Yudin S G, Zlatkin A 1998 Nature 391 874
 Google Scholar
Google Scholar
[13] Blinov L M, Fridkin V M, Palto S P, Bune A V, Dowben P A, Ducharme S 2000 Phys. Usp. 43 243
 Google Scholar
Google Scholar
[14] Mehta R R, Silverman B D, Jacobs J T 1973 J. Appl. Phys. 44 3379
 Google Scholar
Google Scholar
[15] Fong D D, Stephenson G B, Streiffer S K, Eastman J A, Auciello O, Fuoss P H, Thompson C 2004 Science 304 1650
 Google Scholar
Google Scholar
[16] Gruverman A, Wu D, Lu H, Wang Y, Jang H W, Folkman C M, Zhuravlev M Y, Felker D, Rzchowski M, Eom C B, Tsymbal E Y 2009 Nano Lett. 9 3539
 Google Scholar
Google Scholar
[17] Jin Hu W, Wang Z, Yu W, Wu T 2016 Nat. Commun. 7 10808
 Google Scholar
Google Scholar
[18] Wang H, Liu Z R, Yoong H Y, Paudel T R, Xiao J X, Guo R, Lin W N, Yang P, Wang J, Chow G M, Venkatesan T, Tsymbal E Y, Tian H, Chen J S 2018 Nat. Commun. 9 3319
 Google Scholar
Google Scholar
[19] Chang K, Liu J, Lin H, Wang N, Zhao K, Zhang A, Jin F, Zhong Y, Hu X, Duan W, Zhang Q, Fu L, Xue Q K, Chen X, Ji S H 2016 Science 353 274
 Google Scholar
Google Scholar
[20] Yang Q, Wu M, Li J 2018 J. Phys. Chem. Lett. 9 7160
 Google Scholar
Google Scholar
[21] Fei R, Kang W, Yang L 2016 Phys. Rev. Lett. 117 097601
 Google Scholar
Google Scholar
[22] Wu M, Zeng X C 2016 Nano Lett. 16 3236
 Google Scholar
Google Scholar
[23] Liu C, Wan W, Ma J, Guo W, Yao Y 2018 Nanoscale 10 7984
 Google Scholar
Google Scholar
[24] Liu F, You L, Seyler K L, Li X, Yu P, Lin J, Wang X, Zhou J, Wang H, He H, Pantelides S T, Zhou W, Sharma P, Xu X, Ajayan P M, Wang J, Liu Z 2016 Nat. Commun. 7 12357
 Google Scholar
Google Scholar
[25] Xu B, Xiang H, Xia Y, Jiang K, Wan X, He J, Yin J, Liu Z 2017 Nanoscale 9 8427
 Google Scholar
Google Scholar
[26] Reimers J R, Tawfik S A, Ford M J 2018 Chem. Sci. 9 7620
 Google Scholar
Google Scholar
[27] Lai Y, Song Z, Wan Y, Xue M, Wang C, Ye Y, Dai L, Zhang Z, Yang W, Du H, Yang J 2019 Nanoscale 11 5163
 Google Scholar
Google Scholar
[28] Ding W, Zhu J, Wang Z, Gao Y, Xiao D, Gu Y, Zhang Z, Zhu W 2017 Nat. Commun. 8 14956
 Google Scholar
Google Scholar
[29] Cui C, Hu W J, Yan X, Addiego C, Gao W, Wang Y, Wang Z, Li L, Cheng Y, Li P, Zhang X, Alshareef H N, Wu T, Zhu W, Pan X, Li L J 2018 Nano Lett. 18 1253
 Google Scholar
Google Scholar
[30] Wu M, Zeng X C 2017 Nano Lett. 17 6309
 Google Scholar
Google Scholar
[31] Ren Y, Dong S, Wu M 2018 ACS Appl. Mater. Interfaces 10 35361
 Google Scholar
Google Scholar
[32] Wu M, Duan T, Lu C, Fu H, Dong S, Liu J 2018 Nanoscale 10 9509
 Google Scholar
Google Scholar
[33] Valasek J 1921 Phys. Rev. 17 475
 Google Scholar
Google Scholar
[34] Furukawa T 1989 Phase Transitions 18 143
 Google Scholar
Google Scholar
[35] Naber R C, Asadi K, Blom P W, de Leeuw D M, de Boer B 2010 Adv. Mater. 22 933
 Google Scholar
Google Scholar
[36] Heremans P, Gelinck G H, Muller R, Baeg K J, Kim D Y, Noh Y Y 2011 Chem. Mater. 23 341
 Google Scholar
Google Scholar
[37] Tang Y Y, Li P F, Zhang W Y, Ye H Y, You Y M, Xiong R G 2017 J. Am. Chem. Soc. 139 13903
 Google Scholar
Google Scholar
[38] Yang Q, Xiong W, Zhu L, Gao G, Wu M 2017 J. Am. Chem. Soc. 139 11506
 Google Scholar
Google Scholar
[39] Yang C K, Chen W N, Ding Y T, Wang J, Rao Y, Liao W Q, Tang Y Y, Li P F, Wang Z X, Xiong R G 2019 Adv. Mater. 3 1
 Google Scholar
Google Scholar
[40] Shi P P, Lu S Q, Song X J, Chen X G, Liao W Q, Li P F, Tang Y Y, Xiong R G 2019 J. Am. Chem. Soc. 141 18334
 Google Scholar
Google Scholar
[41] Ma L, Jia Y, Ducharme S, Wang J, Zeng X C 2019 J. Am. Chem. Soc. 141 1452
 Google Scholar
Google Scholar
[42] Kresse G, Furthmuller J 1996 Phys. Rev. B 54 11169
 Google Scholar
Google Scholar
[43] Perdew J P, Yue W 1986 Phys. Rev. B 33 8800
 Google Scholar
Google Scholar
[44] Perdew J P, Burke K, Ernzerhof M 1996 Phys. Rev. Lett. 77 3865
 Google Scholar
Google Scholar
[45] Blochl P E 1994 Phys. Rev. B 50 17953
 Google Scholar
Google Scholar
[46] Grimme S, Antony J, Ehrlich S, Krieg H 2010 J. Chem. Phys. 132 154104
 Google Scholar
Google Scholar
[47] Monkhorst H J, Pack J D 1976 Phys. Rev. B 13 5188
 Google Scholar
Google Scholar
[48] King-Smith R D, Vanderbilt D 1993 Phys. Rev. B. 47 1651
 Google Scholar
Google Scholar
[49] Horiuchi S, Tokunaga Y, Giovannetti G, Picozzi S, Itoh H, Shimano R, Kumai R, Tokura Y 2010 Nature 463 789
 Google Scholar
Google Scholar
[50] Horiuchi S, Kumai R, Tokura Y 2011 Adv. Mater. 23 2098
 Google Scholar
Google Scholar
[51] Horiuchi S, Kagawa F, Hatahara K, Kobayashi K, Kumai R, Murakami Y, Tokura Y 2012 Nat. Commun. 3 1308
 Google Scholar
Google Scholar
[52] Horiuchi S, Kobayashi K, Kumai R, Ishibashi S 2017 Nat. Commun. 8 14426
 Google Scholar
Google Scholar
-
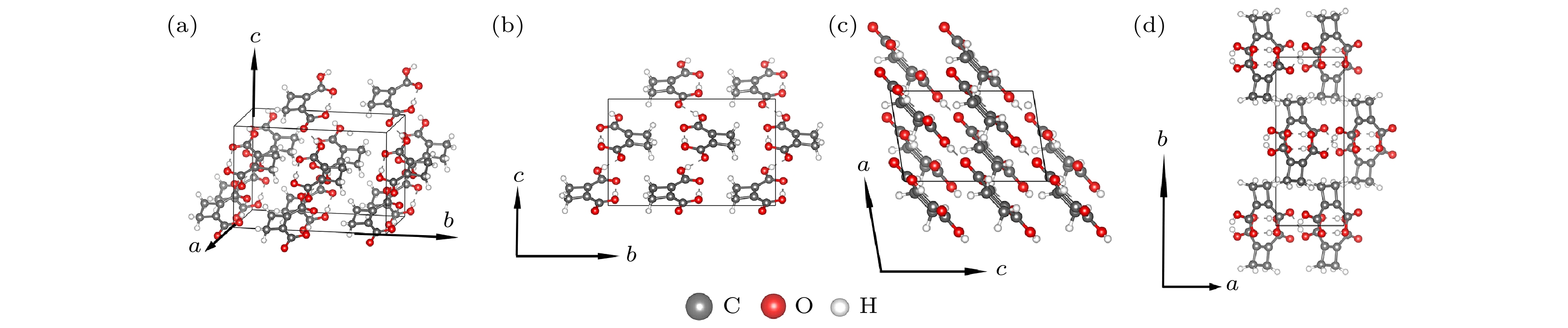
图 1 优化后的CBDC分子晶体结构图示 (a) CBDC块体的斜视图; (b)—(d)分别为晶胞沿a, b, c基矢方向的视图. 图中灰色、白色和红色小球分别指代碳原子、氢原子和氧原子
Fig. 1. Optimized structure of bulk of CBDC molecular crystal: (a) Perspective view of bulk CBDC; (b)–(d) view of bulk CBDC along a, b and c axis, respectively. The gray, white and red balls denote C, H and O atoms, respectively.
图 2 优化后的块体3-HPLN分子沿晶胞不同基矢方向的晶体结构视图 (a) 基矢a方向; (b) 基矢b方向; (c) 基矢c方向. 图中灰色、乳白色和红色小球分别指代碳原子、氢原子和氧原子
Fig. 2. View of optimized structure of bulk 3-HPLN molecular crystal along different axes: (a) Along a axis; (b) along b axis; (c) along c axis. The gray, milk white and red balls denote C, H and O atoms, respectively.
图 3 优化后的块体PhMDA分子沿晶胞不同基矢方向的晶体结构视图 (a) 基矢a方向; (b) 基矢b方向; (c) 基矢c方向. 图中灰色、乳白色和红色小球分别指代碳原子、氢原子和氧原子
Fig. 3. View of optimized structure of bulk PhMDA molecular crystal along different axes:. (a) Along a axis; (b) along b axis; (c) along c axis. The gray, milk white and red balls denote C, H and O atoms respectively.
图 4 CBDC晶体的铁电机制. 图中绿色圆圈标注了氢键的位置, 其中质子的集体迁移导致了铁电极化由晶格
$[20\bar{1}]$ 方向转为$ [\bar{2}01] $ 方向; 灰色、乳白色和红色小球分别指代碳原子、氢原子和氧原子Fig. 4. Ferroelectric mechanism of CBDC crystal. Green circles mark the collective transfer of protons within the hydrogen bonds, leading to the orientation of polarity being reversed from
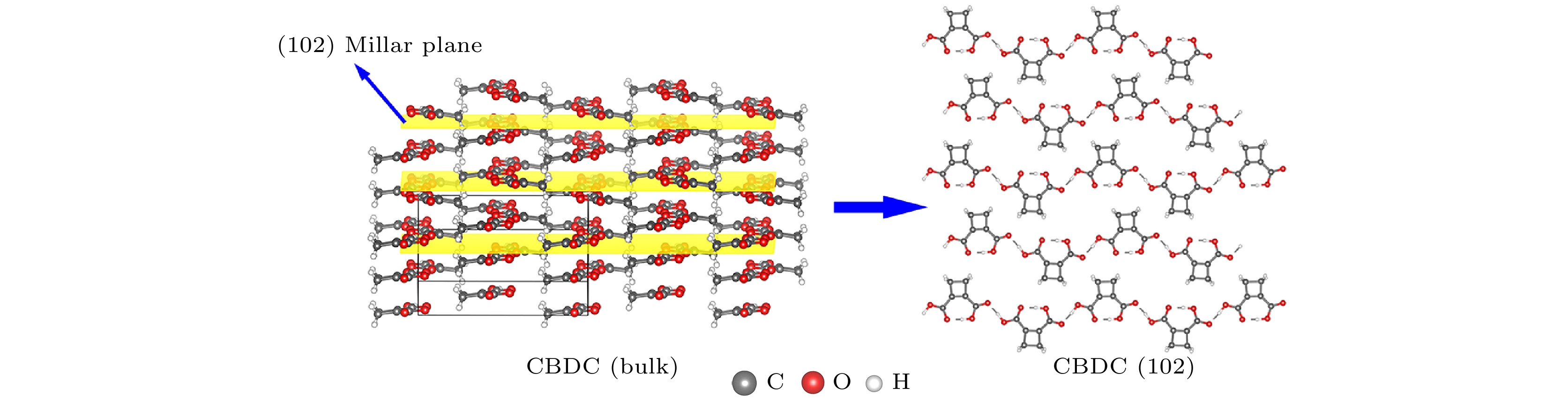
$ [20\bar{1}] $ direction to$ [\bar{2}01] $ direction. The gray, milk white and red balls denote C, H and O atoms, respectively.图 5 CBDC分子晶体块体沿(102)晶面的剥离示意图. 其中左图中黄色平面代表(102)密勒晶面, 右图为(102)单层的顶视图. 灰色乳白色和红色小球分别指代碳原子、氢原子和氧原子.
Fig. 5. Exfoliation of CBDC bulk crystal along the (102) Millar plane. The yellow plane in left panel denotes the (102) plane. The right panel is the top view of the single-unit-thick (102) plane. The gray, white and red balls denote C, H and O atoms respectively.
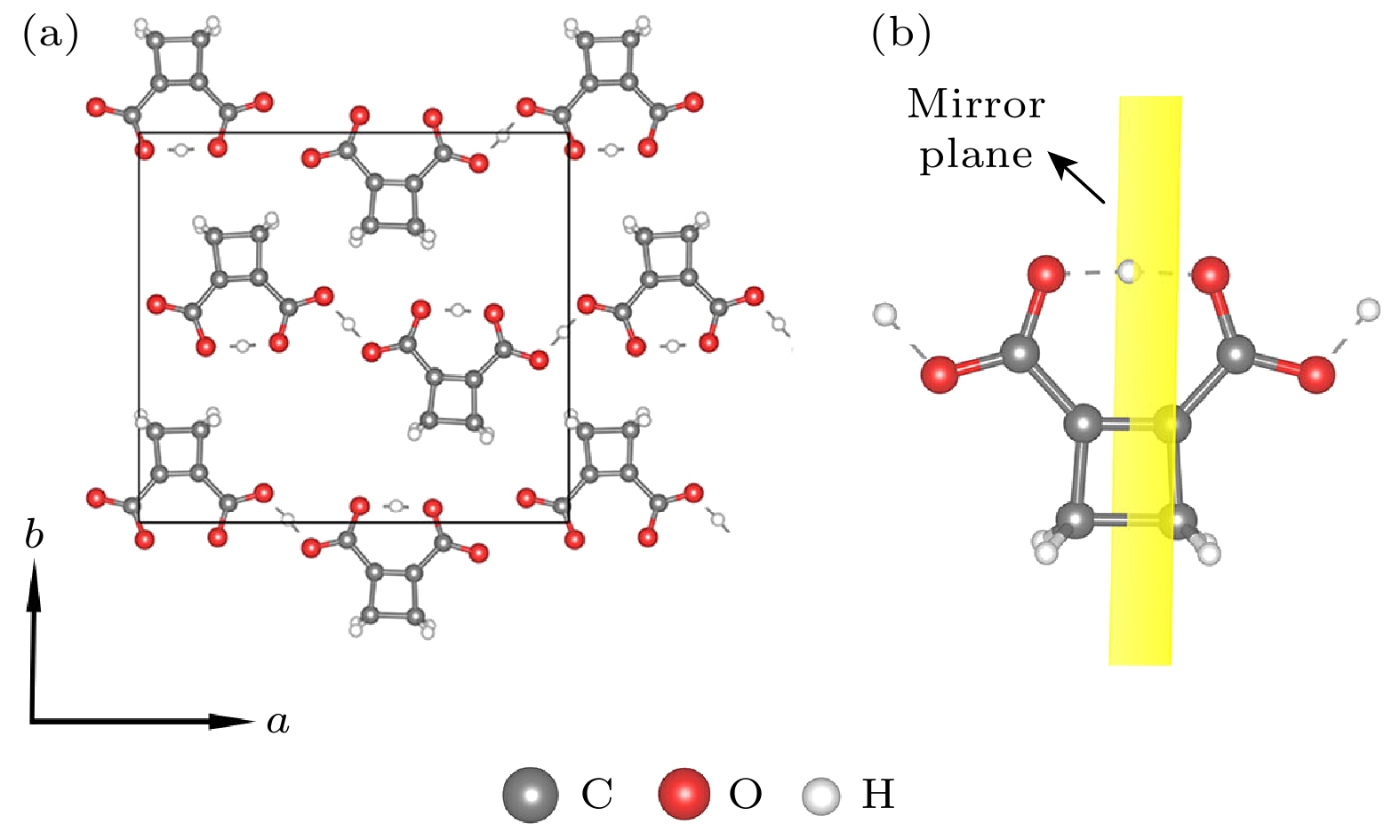
图 7 (a) 顺电相下的CBDC (102), 绿色圆圈中质子处于相邻两个氧原子中间处; (b) 顺电相下的CBDC (102)中的CBDC分子结构示意图, 黄色平面为分子的对称面
Fig. 7. (a) Paraelectric phase of CBDC (102) monolayer crystal, the protons in green circles are located at the center of the adjacent oxygen atoms; (b) CBDC molecular in paraelectric phase, the yellow plane denotes symmetry plane of the molecular.
图 14 石墨烯基底上的CBDC (102)单层分子晶体经过5 ps从头算分子动力学模拟后的结构图(NPT系综, 温度300 K, 时间步长1 fs) (a) 正视图; (b) 侧视图. 图(a)中绿色曲线指示了多条有序排列的氢键链, 为清晰起见, 图(a)中的石墨烯衬底已被隐去
Fig. 14. (a) Top and (b) side views of snapshot of the two-dimensional CBDC (102) monolayer supported on a graphene sheet, after 5 ps of AIMD simulation. The substrate in panel (a) is removed for clarity.
表 1 铁电相CBDC分子晶体的自发极化
Table 1. Spontaneous polarization of ferroelectric phase of CBDC molecular crystal.
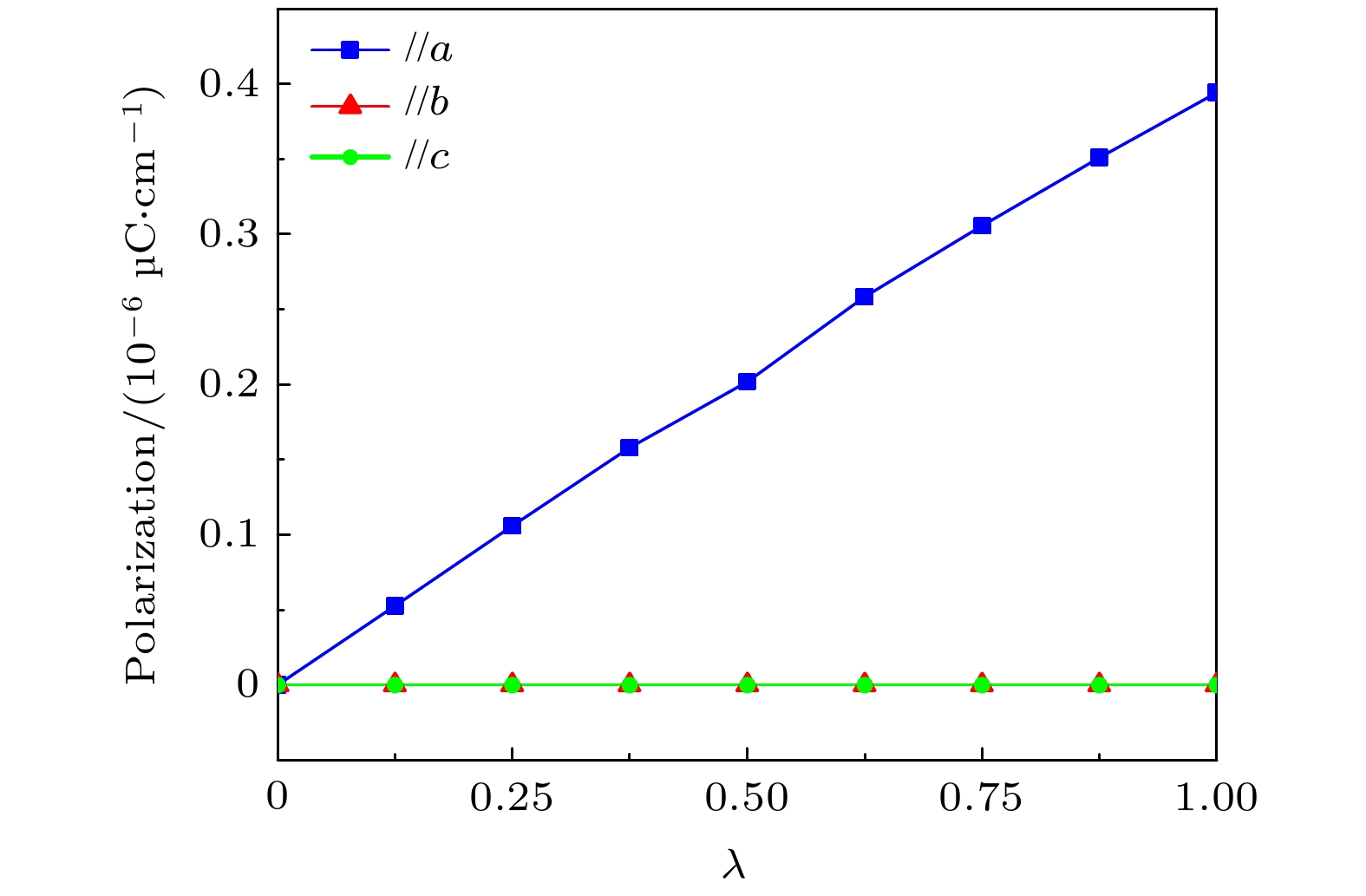
Polarization/(μC·cm–2) a b c Exp. 8.6 0 10 DFT (Horiuchi[52]) 11.7 0 9.6 DFT (Our work) 11.7 0 9.5 表 2 CBDC (102)的晶格参数以及自发铁电极化值
Table 2. Lattice parameters and spontaneous ferroelectric polarization of CBDC (102).
a/Å b/Å γ/(°) Ps/(10–6 μC·cm–1) CBDC (102) 14.92 13.56 90 0.39 -
[1] Novoselov K S, Geim A K, Morozov S V, Jiang D, Zhang Y, Dubonos S V, Grigorieva I V, Firsov A A 2004 Science 306 666
 Google Scholar
Google Scholar
[2] Radisavljevic B, Radenovic A, Brivio J, Giacometti V, Kis A 2011 Nat. Nanotechnol. 6 147
 Google Scholar
Google Scholar
[3] Lopez-Sanchez O, Lembke D, Kayci M, Radenovic A, Kis A 2013 Nat. Nanotechnol. 8 497
 Google Scholar
Google Scholar
[4] Zeng H, Dai J, Yao W, Xiao D, Cui X 2012 Nat. Nanotechnol. 7 490
 Google Scholar
Google Scholar
[5] Cong C, Shang J, Wu X, Cao B, Peimyoo N, Qiu C, Sun L, Yu T 2014 Adv. Opt. Mater. 2 131
 Google Scholar
Google Scholar
[6] Bertolazzi S, Brivio J, Kis A 2011 ACS Nano 5 9703
 Google Scholar
Google Scholar
[7] Cai Y, Lan J, Zhang G, Zhang Y W 2014 Phys. Rev. B 89 035438
 Google Scholar
Google Scholar
[8] Profeta G, Calandra M, Mauri F 2012 Nat. Phys. 8 131
 Google Scholar
Google Scholar
[9] Zhou J J, Feng W, Liu C C, Guan S, Yao Y 2014 Nano Lett. 14 4767
 Google Scholar
Google Scholar
[10] Li X, Yang J 2014 J. Mater. Chem. C 8 8098
 Google Scholar
Google Scholar
[11] Belianinov A, He Q, Dziaugys A, Maksymovych P, Eliseev E, Borisevich A, Morozovska A, Banys J, Vysochanskii Y, Kalinin S V 2015 Nano Lett. 15 3808
 Google Scholar
Google Scholar
[12] Bune A V, Fridkin V M, Ducharme S, Blinov L M, Palto S P, Sorokin A V, Yudin S G, Zlatkin A 1998 Nature 391 874
 Google Scholar
Google Scholar
[13] Blinov L M, Fridkin V M, Palto S P, Bune A V, Dowben P A, Ducharme S 2000 Phys. Usp. 43 243
 Google Scholar
Google Scholar
[14] Mehta R R, Silverman B D, Jacobs J T 1973 J. Appl. Phys. 44 3379
 Google Scholar
Google Scholar
[15] Fong D D, Stephenson G B, Streiffer S K, Eastman J A, Auciello O, Fuoss P H, Thompson C 2004 Science 304 1650
 Google Scholar
Google Scholar
[16] Gruverman A, Wu D, Lu H, Wang Y, Jang H W, Folkman C M, Zhuravlev M Y, Felker D, Rzchowski M, Eom C B, Tsymbal E Y 2009 Nano Lett. 9 3539
 Google Scholar
Google Scholar
[17] Jin Hu W, Wang Z, Yu W, Wu T 2016 Nat. Commun. 7 10808
 Google Scholar
Google Scholar
[18] Wang H, Liu Z R, Yoong H Y, Paudel T R, Xiao J X, Guo R, Lin W N, Yang P, Wang J, Chow G M, Venkatesan T, Tsymbal E Y, Tian H, Chen J S 2018 Nat. Commun. 9 3319
 Google Scholar
Google Scholar
[19] Chang K, Liu J, Lin H, Wang N, Zhao K, Zhang A, Jin F, Zhong Y, Hu X, Duan W, Zhang Q, Fu L, Xue Q K, Chen X, Ji S H 2016 Science 353 274
 Google Scholar
Google Scholar
[20] Yang Q, Wu M, Li J 2018 J. Phys. Chem. Lett. 9 7160
 Google Scholar
Google Scholar
[21] Fei R, Kang W, Yang L 2016 Phys. Rev. Lett. 117 097601
 Google Scholar
Google Scholar
[22] Wu M, Zeng X C 2016 Nano Lett. 16 3236
 Google Scholar
Google Scholar
[23] Liu C, Wan W, Ma J, Guo W, Yao Y 2018 Nanoscale 10 7984
 Google Scholar
Google Scholar
[24] Liu F, You L, Seyler K L, Li X, Yu P, Lin J, Wang X, Zhou J, Wang H, He H, Pantelides S T, Zhou W, Sharma P, Xu X, Ajayan P M, Wang J, Liu Z 2016 Nat. Commun. 7 12357
 Google Scholar
Google Scholar
[25] Xu B, Xiang H, Xia Y, Jiang K, Wan X, He J, Yin J, Liu Z 2017 Nanoscale 9 8427
 Google Scholar
Google Scholar
[26] Reimers J R, Tawfik S A, Ford M J 2018 Chem. Sci. 9 7620
 Google Scholar
Google Scholar
[27] Lai Y, Song Z, Wan Y, Xue M, Wang C, Ye Y, Dai L, Zhang Z, Yang W, Du H, Yang J 2019 Nanoscale 11 5163
 Google Scholar
Google Scholar
[28] Ding W, Zhu J, Wang Z, Gao Y, Xiao D, Gu Y, Zhang Z, Zhu W 2017 Nat. Commun. 8 14956
 Google Scholar
Google Scholar
[29] Cui C, Hu W J, Yan X, Addiego C, Gao W, Wang Y, Wang Z, Li L, Cheng Y, Li P, Zhang X, Alshareef H N, Wu T, Zhu W, Pan X, Li L J 2018 Nano Lett. 18 1253
 Google Scholar
Google Scholar
[30] Wu M, Zeng X C 2017 Nano Lett. 17 6309
 Google Scholar
Google Scholar
[31] Ren Y, Dong S, Wu M 2018 ACS Appl. Mater. Interfaces 10 35361
 Google Scholar
Google Scholar
[32] Wu M, Duan T, Lu C, Fu H, Dong S, Liu J 2018 Nanoscale 10 9509
 Google Scholar
Google Scholar
[33] Valasek J 1921 Phys. Rev. 17 475
 Google Scholar
Google Scholar
[34] Furukawa T 1989 Phase Transitions 18 143
 Google Scholar
Google Scholar
[35] Naber R C, Asadi K, Blom P W, de Leeuw D M, de Boer B 2010 Adv. Mater. 22 933
 Google Scholar
Google Scholar
[36] Heremans P, Gelinck G H, Muller R, Baeg K J, Kim D Y, Noh Y Y 2011 Chem. Mater. 23 341
 Google Scholar
Google Scholar
[37] Tang Y Y, Li P F, Zhang W Y, Ye H Y, You Y M, Xiong R G 2017 J. Am. Chem. Soc. 139 13903
 Google Scholar
Google Scholar
[38] Yang Q, Xiong W, Zhu L, Gao G, Wu M 2017 J. Am. Chem. Soc. 139 11506
 Google Scholar
Google Scholar
[39] Yang C K, Chen W N, Ding Y T, Wang J, Rao Y, Liao W Q, Tang Y Y, Li P F, Wang Z X, Xiong R G 2019 Adv. Mater. 3 1
 Google Scholar
Google Scholar
[40] Shi P P, Lu S Q, Song X J, Chen X G, Liao W Q, Li P F, Tang Y Y, Xiong R G 2019 J. Am. Chem. Soc. 141 18334
 Google Scholar
Google Scholar
[41] Ma L, Jia Y, Ducharme S, Wang J, Zeng X C 2019 J. Am. Chem. Soc. 141 1452
 Google Scholar
Google Scholar
[42] Kresse G, Furthmuller J 1996 Phys. Rev. B 54 11169
 Google Scholar
Google Scholar
[43] Perdew J P, Yue W 1986 Phys. Rev. B 33 8800
 Google Scholar
Google Scholar
[44] Perdew J P, Burke K, Ernzerhof M 1996 Phys. Rev. Lett. 77 3865
 Google Scholar
Google Scholar
[45] Blochl P E 1994 Phys. Rev. B 50 17953
 Google Scholar
Google Scholar
[46] Grimme S, Antony J, Ehrlich S, Krieg H 2010 J. Chem. Phys. 132 154104
 Google Scholar
Google Scholar
[47] Monkhorst H J, Pack J D 1976 Phys. Rev. B 13 5188
 Google Scholar
Google Scholar
[48] King-Smith R D, Vanderbilt D 1993 Phys. Rev. B. 47 1651
 Google Scholar
Google Scholar
[49] Horiuchi S, Tokunaga Y, Giovannetti G, Picozzi S, Itoh H, Shimano R, Kumai R, Tokura Y 2010 Nature 463 789
 Google Scholar
Google Scholar
[50] Horiuchi S, Kumai R, Tokura Y 2011 Adv. Mater. 23 2098
 Google Scholar
Google Scholar
[51] Horiuchi S, Kagawa F, Hatahara K, Kobayashi K, Kumai R, Murakami Y, Tokura Y 2012 Nat. Commun. 3 1308
 Google Scholar
Google Scholar
[52] Horiuchi S, Kobayashi K, Kumai R, Ishibashi S 2017 Nat. Commun. 8 14426
 Google Scholar
Google Scholar
计量
- 文章访问数: 7092
- PDF下载量: 120
- 被引次数: 0













 下载:
下载: