-
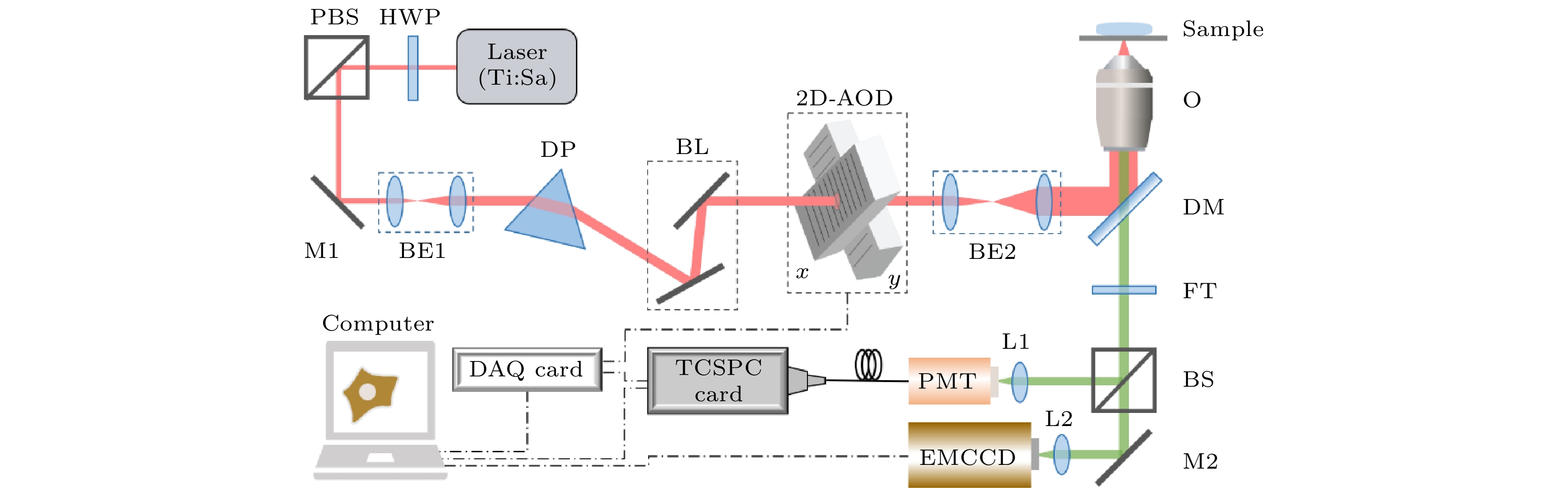
荧光寿命显微成像(fluorescence lifetime imaging microscopy, FLIM)技术在细胞微环境传感中具有特异性强、灵敏度高、可定量的优点, 被广泛应用于生物医学研究. 其中, 基于时间相关单光子计数(time-correlated single photon counting, TCSPC)进行荧光寿命探测的方法是目前最常用的技术之一, 但受成像原理和条件限制, 该技术存在数据采集时间较长、成像速度不够快的不足. 本文开发一种能对生物样品中任意数量离散的、形状不规则的感兴趣区域(region of interest, ROI)进行快速FLIM成像的技术. 该技术利用声光偏转器(acousto-optic deflector, AOD)实现快速灵活的寻址扫描, 并通过对ROI形状特征的简单在线分析, 实现AOD与TCSPC同步策略的优化及寿命图像的准确重构, 对于生物样品中常见的存在多个离散不规则ROI情形, 可大幅节省数据采集时间, 从而实现对这些ROI的快速FLIM成像. 采用该技术, 对氯化铵刺激下活细胞中溶酶体探针LysoSensor Green DND-189的荧光寿命变化进行了动态FLIM成像, 以监测溶酶体管腔内pH值的实时变化情况. 结果表明, 该快速FLIM技术可用于动态监测生物样品中微环境的变化, 将在活细胞微环境传感中发挥重要作用.
-
关键词:
- 快速荧光寿命显微成像 /
- 寻址扫描 /
- 离散目标 /
- 不规则感兴趣区域
Fluorescence lifetime imaging microscopy (FLIM) has been widely used in biomedical research due to its high specificity, high sensitivity and quantification ability in cell microenvironment sensing. The fluorescence lifetime detection method based on time-correlated single photon counting (TCSPC) is one of the most commonly used techniques at present. However, due to the limitation of imaging principles and conditions, this technique has the disadvantages of long data acquisition time and consequently low imaging speed. In this paper, a fast FLIM technique for any number of discrete and irregular regions of interest (ROIs) in biological samples is developed. The technology uses acousto-optic deflectors (AODs) to achieve fast and flexible addressing scanning, optimize the synchronization strategy between AOD and TCSPC, and reconstruct the lifetime image through simple online feature analysis of the ROI shapes. For the case of multiple discrete irregular ROIs in biological samples, it can greatly save the time of data acquisition, thus realizing the fast FLIM imaging of these ROIs, which is benificial to the study of the heterogeneity of biological events in biological system. In particular, the fast fluorescence imaging result for 87 discrete points in the field of view shows that this method can obtain a fluorescence lifetime image in a very short acquisition time (only 52.2 ms) and thus achieving a very fast imaging speed in such a situation. Dynamic FLIM imaging of lysosome probe LysoSensor Green DND-189 in living cells stimulated by ammonium chloride is carried out to monitor the real-time change of pH value in lysosome lumen. The acquisition time for a single fluorescence lifetime image of lysosomes in two ROIs is only 200 ms. The results show that the rapid FLIM technology can be used to dynamically monitor the changes of microenvironment in biological samples, and will play an important role in the microenvironment sensing in living cells.-
Keywords:
- fast fluorescence lifetime imaging microscopy /
- addressing scanning /
- discrete target /
- irregular regions of interest
[1] Becker W 2012 J. Microsc. 247 119
 Google Scholar
Google Scholar
[2] 刘雄波, 林丹樱, 吴茜茜, 严伟, 罗腾, 杨志刚, 屈军乐 2018 67 178701
 Google Scholar
Google Scholar
Liu X B, Lin D Y, Wu Q Q, Yan W, Luo T, Yang Z G, Qu J L 2018 Acta Phys. Sin. 67 178701
 Google Scholar
Google Scholar
[3] Bower A J, Li J, Chaney E J, Marjanovic M, Spillman D R, Boppart S A 2018 Optica 5 1290
 Google Scholar
Google Scholar
[4] Datta R, Heaster T M, Sharick J T, Gillette A A, Skala M C 2020 J. Biomed. Opt. 25 071203
 Google Scholar
Google Scholar
[5] Hirvonen L M, Suhling K 2020 Front. Phys. 8 161
 Google Scholar
Google Scholar
[6] Suhling K, Hirvonen L M, Levitt J A, Chung P H, Tregidgo C, Marois A L, Rusakov D A, Zheng K, Ameer-Beg S, Poland S, Coelho S, Henderson R, Krstajic N 2015 Med. Photon. 27 3
 Google Scholar
Google Scholar
[7] Liu X B, Lin D Y, Becker W, Niu J J, Yu B, Liu L W, Qu J L 2019 J. Innov. Opt. Health Sci. 12 1930003
 Google Scholar
Google Scholar
[8] Ranjit S, Malacrida L, Stakic M, Gratton E 2019 J. Biophotonics. 12 e201900156
 Google Scholar
Google Scholar
[9] 林丹樱, 牛敬敬, 刘雄波, 张潇, 张娇, 于斌, 屈军乐 2020 69 168703
 Google Scholar
Google Scholar
Lin D Y, Niu J J, Liu X B, Zhang X, Zhang J, Yu B, Qu J L 2020 Acta Phys. Sin. 69 168703
 Google Scholar
Google Scholar
[10] Cominelli A, Acconcia G, Peronio P, Ghioni M, Rech I 2017 Rev. Sci. Instrum. 88 123701
 Google Scholar
Google Scholar
[11] Vallmitjana A, Torrado B, Dvorbikov A, Ranjit S, Gratton E 2020 J. Phys. Chem. B 124 10126
 Google Scholar
Google Scholar
[12] Hirmiz N, Tsikouras A, Osterlund E J, Richards M, Andrews D W, Fang Q 2021 Opt. Lett. 45 69
 Google Scholar
Google Scholar
[13] Wang L, Liang X, Mohammed Y H, Thomas J A, Bridle K R, Thorling C A, Grice J E, Xu Z P, Liu X, Crawford D H G, Roberts M S 2015 Biomed. Opt. Express 6 780
 Google Scholar
Google Scholar
[14] Grewe B F, Langer D, Kasper H, Kampa B M, Helmchen F 2010 Nat. Methods 7 399
 Google Scholar
Google Scholar
[15] Gershow M H, Karagyozov D, Yamaguchi A 2021 Opt. Lett. 46 1644
 Google Scholar
Google Scholar
[16] Qi J, Shao Y H, Liu L X, Wang K G, Chen T S, Qu J L, Niu H B 2013 Opt. Lett. 38 1697
 Google Scholar
Google Scholar
[17] Yan W, Peng X, Qi J, Gao J, Fan S P, Wang Q, Qu J L, Niu H B 2014 J. Biomed. Opt. 19 116004
 Google Scholar
Google Scholar
[18] Zeng S, Lv X, Bi K, Zhan C, Li D, Chen W R, Xiong W, Jacques S L, Luo Q 2007 J. Biomed. Opt. 12 024015
 Google Scholar
Google Scholar
[19] Wu Q Q, Qi J, Lin D Y, Yan W, Hu R, Peng X, Qu J L 2017 Proc. SPIE 10069 1006922
 Google Scholar
Google Scholar
[20] Lin H J, Herman P, Kang J S, Lakowicz J R 2001 Anal. Biochem. 294 118
 Google Scholar
Google Scholar
-
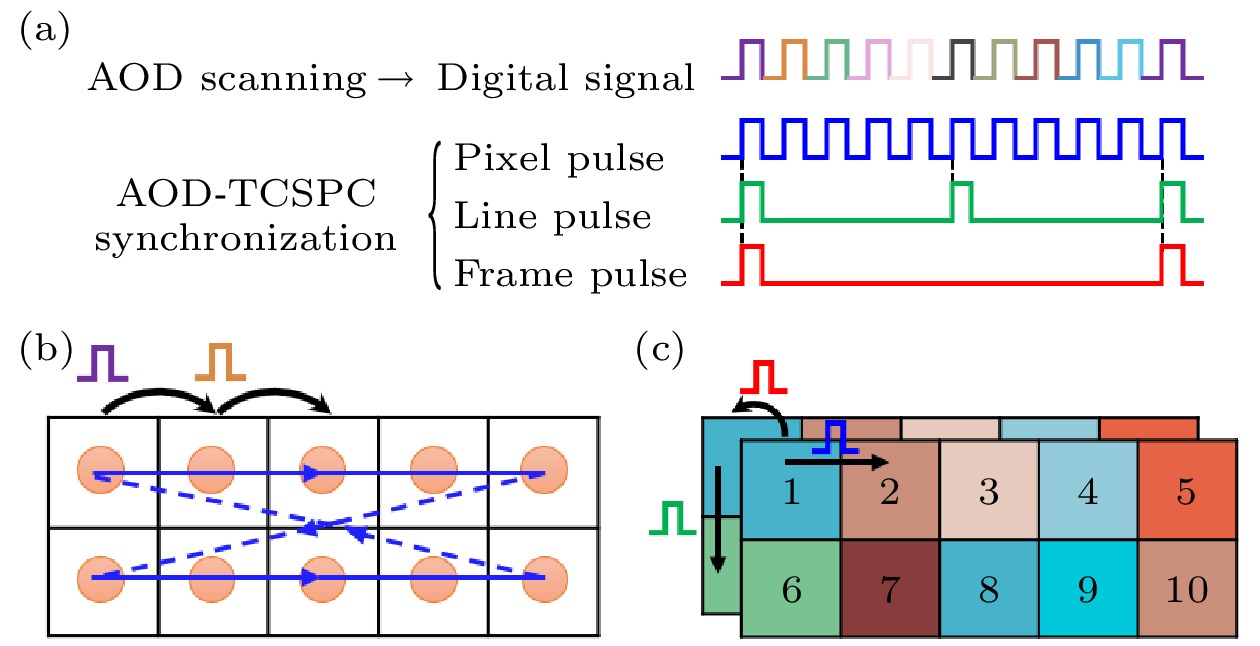
图 3 针对任意数量离散不规则ROI的同步信号产生新策略示意图 (a)三个离散的不同形状ROI(灰色); (b)每个扫描点所需的同步信号; (c)按新策略产生的三路同步信号; (d)已分行存储但仍未准确映射的荧光寿命数据
Fig. 3. Schematic diagram of a new strategy for generating synchronization signals for any number of discrete irregular ROIs: (a) Three discrete ROIs of different shapes (gray); (b) the synchronization signals required for each scan point; (c) three synchronization signals generated according to the new strategy; (d) fluorescence lifetime image data that has been stored in rows before mapping.
图 4 寿命数据处理方法示意图 (a)扫描ROI; (b)外接矩形; (c)二值化矩阵; (d)二值化矩阵非零元素列坐标; (e)寿命数据顺序存储列坐标; (f) X方向移位矩阵; (g)二值化矩阵非零元素行坐标; (h)寿命数据顺序存储行坐标; (i) Y方向移位矩阵; (j)未移位的寿命图像; (k) X方向移位后的寿命图像; (l) Y方向移位后的准确寿命图像
Fig. 4. Schematic diagram of the processing method for the lifetime image data: (a) Scanned ROIs; (b) circumscribed rectangle; (c) binarized matrix; (d) column coordinates of non-zero elements in the binarized matrix; (e) column coordinates of the lifetime data before mapping; (f) shift matrix in X-direction; (g) row coordinates of non-zero elements in the binarized matrix; (h) row coordinates of the lifetime data before mapping; (i) shift matrix in Y-direction; (j) lifetime image before mapping; (k) lifetime image after shift in X-direction; (l) correct lifetime image after shift in Y-direction.
图 5 铃兰根茎切片样品多个离散的不同形状ROI的FLIM成像 (a)明场图像及ROI的选取; (b) EMCCD采集的荧光强度图像; (c) TCSPC采集得到的寿命图像; (d)准确重构的荧光寿命图像
Fig. 5. FLIM imaging of multiple discrete ROIs of different shapes in convallaria slice sample: (a) Bright field image collected by EMCCD and selection of ROIs; (b) fluorescence intensity image collected by EMCCD; (c) lifetime image collected by TCSPC; (d) corrected fluorescence lifetime image.
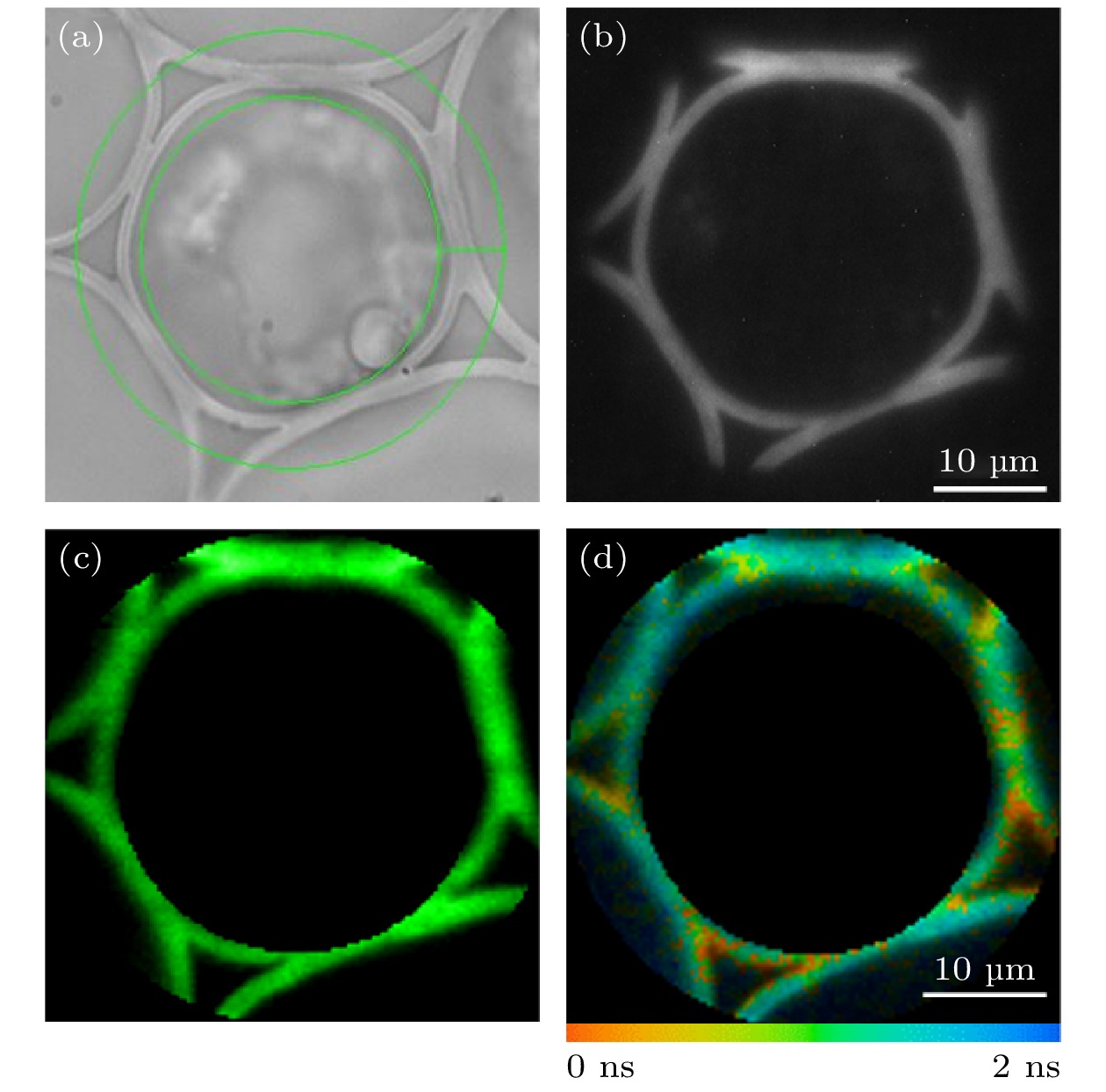
图 6 铃兰根茎切片样品环形ROI的FLIM成像 (a)环形ROI的选取; (b) EMCCD采集的荧光强度图像; (c) TCSPC采集的荧光强度图像; (d)荧光寿命图像
Fig. 6. FLIM imaging of a circular ROI in convallaria slice sample: (a) Selection of the circular ROI; (b) fluorescence intensity image collected by EMCCD; (c) fluorescence intensity image collected by TCSPC; (d) fluorescence lifetime image.
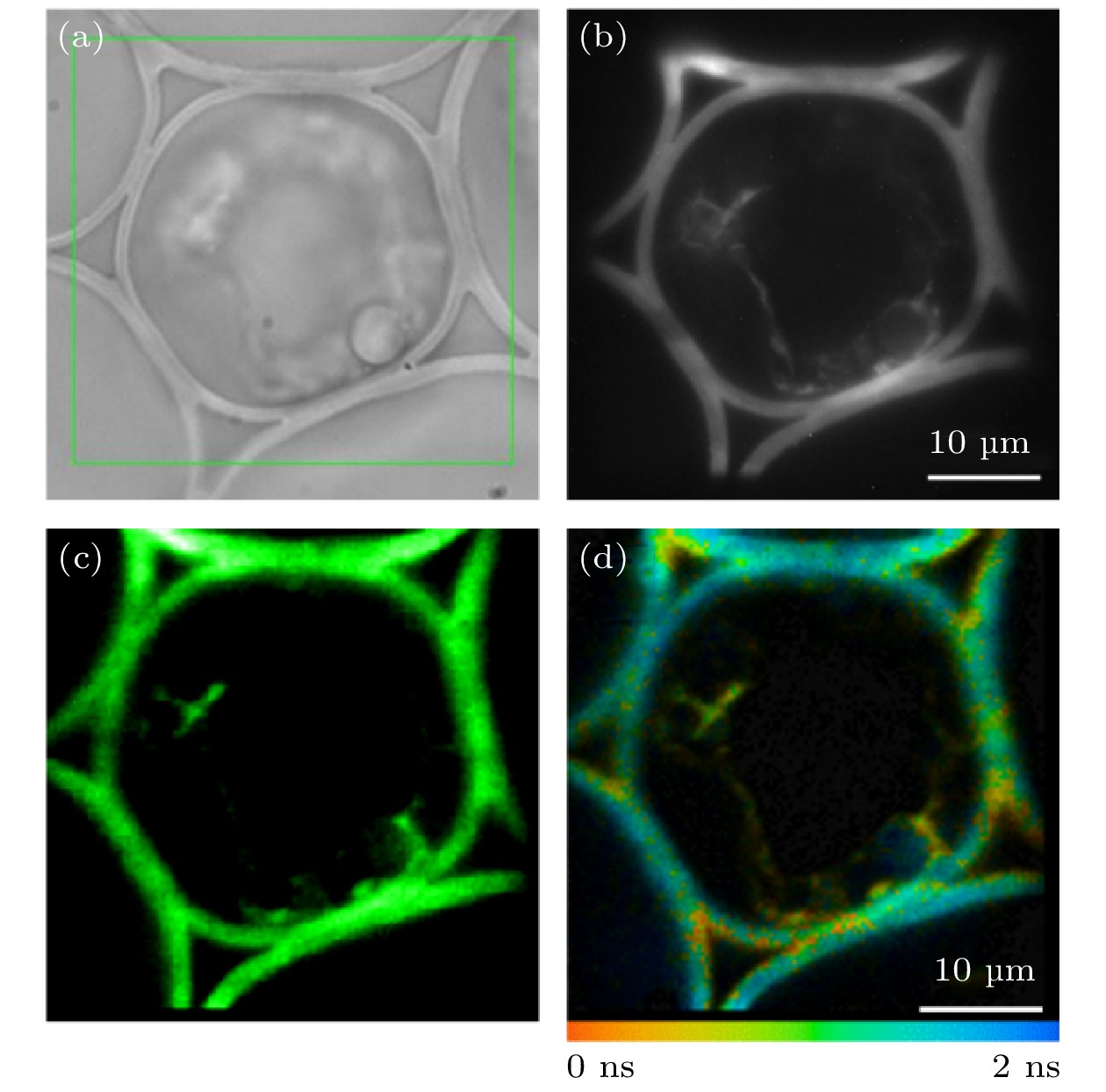
图 7 铃兰根茎切片样品矩形ROI的FLIM成像 (a)矩形ROI的选取; (b) EMCCD采集的荧光强度图像; (c) TCSPC采集的荧光强度图像; (d)荧光寿命图像
Fig. 7. FLIM imaging of a rectangular ROI in convallaria slice sample: (a) Selection of the rectangular ROI; (b) fluorescence intensity image collected by EMCCD; (c) fluorescence intensity image collected by TCSPC; (d) fluorescence lifetime image.
图 8 罗丹明6G溶液样品中离散点的快速FLIM成像 (a)离散像素点的选取; (b) EMCCD采集的荧光强度图像; (c) TCSPC采集的荧光强度图像; (d)荧光寿命图像
Fig. 8. Fast FLIM imaging of discrete pixels in rhodamine 6G solution: (a) Selection of the discrete pixels; (b) fluorescence intensity image collected by EMCCD; (c) fluorescence intensity image collected by TCSPC; (d) fluorescence lifetime image.
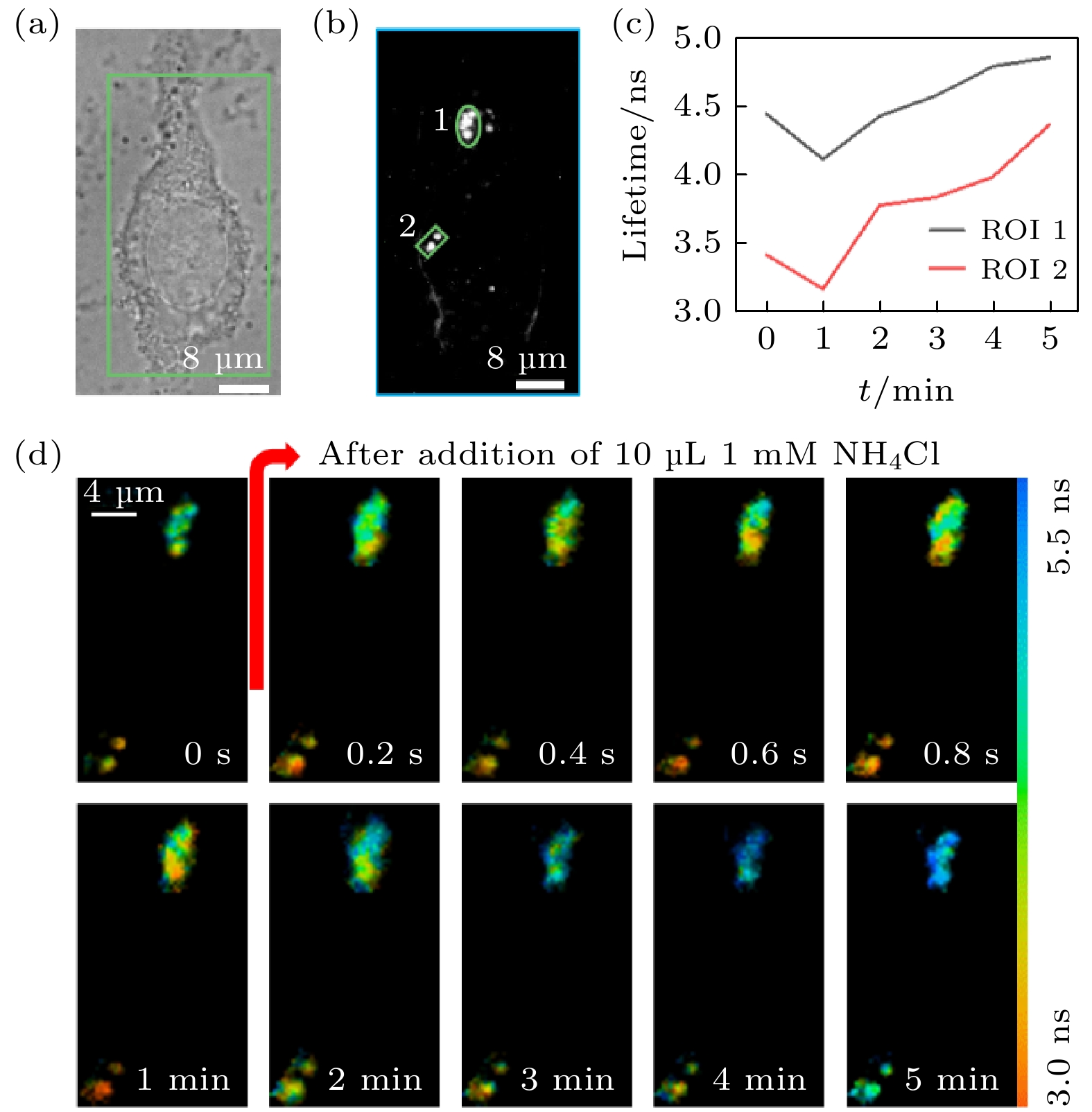
图 9 活细胞中LysoSensor-DND189标记溶酶体的快速FLIM成像 (a)明场图像; (b) EMCCD采集的荧光强度图像及ROI的选取; (c) ROI平均荧光寿命随氯化铵刺激时间的变化; (d)部分时间点的荧光寿命图像
Fig. 9. Fast FLIM imaging of LysoSensor-DND189 labeled lysosomes in living cells: (a) Bright field image; (b) fluorescence intensity image collected by EMCCD and selection of ROIs; (c) the change of the average fluorescence lifetime in the ROIs with the stimulation time of ammonium chloride; (d) the fluorescence lifetime image of selected time points.
-
[1] Becker W 2012 J. Microsc. 247 119
 Google Scholar
Google Scholar
[2] 刘雄波, 林丹樱, 吴茜茜, 严伟, 罗腾, 杨志刚, 屈军乐 2018 67 178701
 Google Scholar
Google Scholar
Liu X B, Lin D Y, Wu Q Q, Yan W, Luo T, Yang Z G, Qu J L 2018 Acta Phys. Sin. 67 178701
 Google Scholar
Google Scholar
[3] Bower A J, Li J, Chaney E J, Marjanovic M, Spillman D R, Boppart S A 2018 Optica 5 1290
 Google Scholar
Google Scholar
[4] Datta R, Heaster T M, Sharick J T, Gillette A A, Skala M C 2020 J. Biomed. Opt. 25 071203
 Google Scholar
Google Scholar
[5] Hirvonen L M, Suhling K 2020 Front. Phys. 8 161
 Google Scholar
Google Scholar
[6] Suhling K, Hirvonen L M, Levitt J A, Chung P H, Tregidgo C, Marois A L, Rusakov D A, Zheng K, Ameer-Beg S, Poland S, Coelho S, Henderson R, Krstajic N 2015 Med. Photon. 27 3
 Google Scholar
Google Scholar
[7] Liu X B, Lin D Y, Becker W, Niu J J, Yu B, Liu L W, Qu J L 2019 J. Innov. Opt. Health Sci. 12 1930003
 Google Scholar
Google Scholar
[8] Ranjit S, Malacrida L, Stakic M, Gratton E 2019 J. Biophotonics. 12 e201900156
 Google Scholar
Google Scholar
[9] 林丹樱, 牛敬敬, 刘雄波, 张潇, 张娇, 于斌, 屈军乐 2020 69 168703
 Google Scholar
Google Scholar
Lin D Y, Niu J J, Liu X B, Zhang X, Zhang J, Yu B, Qu J L 2020 Acta Phys. Sin. 69 168703
 Google Scholar
Google Scholar
[10] Cominelli A, Acconcia G, Peronio P, Ghioni M, Rech I 2017 Rev. Sci. Instrum. 88 123701
 Google Scholar
Google Scholar
[11] Vallmitjana A, Torrado B, Dvorbikov A, Ranjit S, Gratton E 2020 J. Phys. Chem. B 124 10126
 Google Scholar
Google Scholar
[12] Hirmiz N, Tsikouras A, Osterlund E J, Richards M, Andrews D W, Fang Q 2021 Opt. Lett. 45 69
 Google Scholar
Google Scholar
[13] Wang L, Liang X, Mohammed Y H, Thomas J A, Bridle K R, Thorling C A, Grice J E, Xu Z P, Liu X, Crawford D H G, Roberts M S 2015 Biomed. Opt. Express 6 780
 Google Scholar
Google Scholar
[14] Grewe B F, Langer D, Kasper H, Kampa B M, Helmchen F 2010 Nat. Methods 7 399
 Google Scholar
Google Scholar
[15] Gershow M H, Karagyozov D, Yamaguchi A 2021 Opt. Lett. 46 1644
 Google Scholar
Google Scholar
[16] Qi J, Shao Y H, Liu L X, Wang K G, Chen T S, Qu J L, Niu H B 2013 Opt. Lett. 38 1697
 Google Scholar
Google Scholar
[17] Yan W, Peng X, Qi J, Gao J, Fan S P, Wang Q, Qu J L, Niu H B 2014 J. Biomed. Opt. 19 116004
 Google Scholar
Google Scholar
[18] Zeng S, Lv X, Bi K, Zhan C, Li D, Chen W R, Xiong W, Jacques S L, Luo Q 2007 J. Biomed. Opt. 12 024015
 Google Scholar
Google Scholar
[19] Wu Q Q, Qi J, Lin D Y, Yan W, Hu R, Peng X, Qu J L 2017 Proc. SPIE 10069 1006922
 Google Scholar
Google Scholar
[20] Lin H J, Herman P, Kang J S, Lakowicz J R 2001 Anal. Biochem. 294 118
 Google Scholar
Google Scholar
计量
- 文章访问数: 8143
- PDF下载量: 121
- 被引次数: 0













 下载:
下载: