-
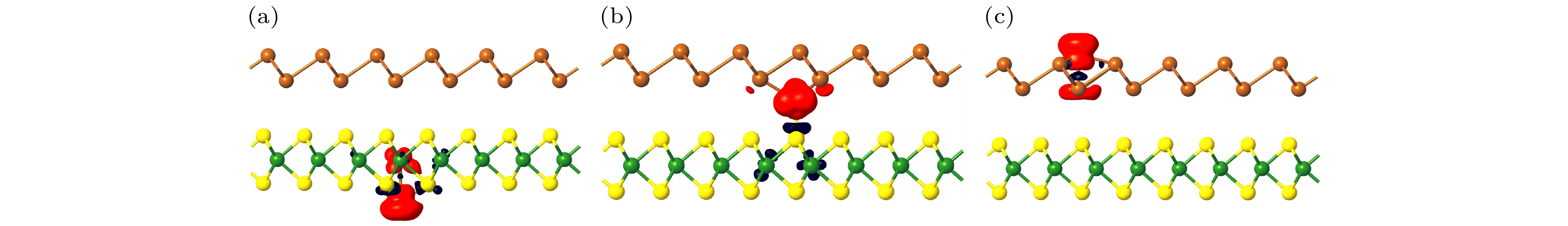
To study the induced magnetism mechanism and magneto-electronic properties of non-magnetic two-dimensional van der Waals heterostructure adsorbing magnetic atoms, we construct Sb/WS2 heterostructure, and consider its adsorbed Fe atoms. The calculated adsorption energy shows that TW, VSb adsorption are the most likely positions for Fe atom adsorbed below and above the heterostructure, respectively, and TS_M adsorption is the most likely position for Fe atom adsorbed between two monolayers. The induced magnetism is due to the electron-spin rearrangement caused by the expansion of valence electronic configuration (VEC) and charge transfer after Fe atoms have been adsorbed. The TW adsorption and the TS_M adsorption make the nonmagnetic semiconducting heterostructure become a half-semiconductor (HSC), while VSb adsorption turns the heterostructure into a bipolar magnetic semiconductor (BMS). In particular, the calculated magnetized energy indicates that the interlayer TS_M adsorption leads the heterostructure to holding the highest magnetic stability, which is enough to resist the influence of thermal fluctuation at room temperature. Quantum manipulation can cause the heterostructure to produce abundant magnetism, especially the flexible change of magnetic phase. For example, the application of external electric field can give rise to the magnetic phase transition among HSC, HM (half-metal) and BMS for the heterostructure, and the vertical strain can make the heterostructure realize the magnetic phase transition among HSC, HM and MM (magnetic metal). This study shows that the heterostructure can increase the adsorption region of transition metal atoms (below, interlayer and above), so as to produce rich magnetism, especially for the interlayer adsorption of transition metals, its magnetic stability against temperature is significantly enhanced.
-
Keywords:
- two-dimensional van der Waals heterostructure /
- magneto-electronic property /
- magnetic stability /
- quantum manipulation /
- magnetic phase transition
[1] Geim A K, Grigorieva I V 2013 Nature 499 419
 Google Scholar
Google Scholar
[2] Li X M, Tao L, Chen Z F, Fang H, Li X S, Wang X R, Xu J B, Zhu H W 2017 Appl. Phys. Rev. 4 021306
 Google Scholar
Google Scholar
[3] Novoselov K S, Mishchenko A, Carvalho A, Neto A H C 2016 Science 353 aac9439
 Google Scholar
Google Scholar
[4] Shang J M, Pan L F, Wang X T, Li J B, Deng H X, Wei Z M 2018 J. Mater. Chem. C 6 7201
 Google Scholar
Google Scholar
[5] Idrees M, Fawad M, Bilal M, Saeed Y, Nguyen C, Amin B 2020 RSC Adv. 10 25801
 Google Scholar
Google Scholar
[6] Ozcelik V O, Azadani J G, Yang C, Koester S J, Low T 2016 Phys. Rev. B 94 035125
 Google Scholar
Google Scholar
[7] Liu C H, Clark G, Fryett T, Wu S F, Zheng J J, Hatami F, Xu X D, Majumdar A 2017 Nano Lett. 17 200
 Google Scholar
Google Scholar
[8] Binder J, Withers F, Molas M R, Faugeras C, Nogajewski K, Watanabe K, Taniguchi T, Kozikov A, Geim A K, Novoselov K S, Potemski M 2017 Nano Lett. 17 1425
 Google Scholar
Google Scholar
[9] He X, Deng X Q, Sun L, Zhang Z H, Fan Z Q 2022 Appl. Surf. Sci. 578 151844
 Google Scholar
Google Scholar
[10] Huang L, Huo N J, Li Y, Chen H, Yang J H, Wei Z M, Li J B, Li S S 2015 J. Phys. Chem. Lett. 6 2483
 Google Scholar
Google Scholar
[11] Xiao W Z, Xu L, Rong Q Y, Dai X Y, Cheng C P, Wang L L 2020 Appl. Surf. Sci. 504 144425
 Google Scholar
Google Scholar
[12] Huang L, Li Y, Wei Z M, Li J B 2015 Sci. Rep. 5 16448
 Google Scholar
Google Scholar
[13] Lei C G, Ma Y D, Xu X L, Zhang T, Huang B B, Dai Y 2019 J. Phys. Chem. C 123 23089
 Google Scholar
Google Scholar
[14] Yan R S, Fathipour S, Han Y M, Song B, Xiao S D, Li M D, Ma N, Protasenko V, Muller D A, Jena D, Xing H G 2015 Nano Lett. 15 5791
 Google Scholar
Google Scholar
[15] Shim J, Oh S, Kang D H, Jo S H, Ali M H, Choi W Y, Heo K, Jeon J, Lee S, Kim M, Song Y J, Park J H 2016 Nat. Commun. 7 13413
 Google Scholar
Google Scholar
[16] Xia C X, Du J, Li M, Li X P, Zhao X, Wang T X, Li J B 2018 Phy. Rev. A 10 054064
 Google Scholar
Google Scholar
[17] Wu Y B, Huang Z Y, Liu H T, He C Y, Xue L, Qi X, Zhong J X 2018 Phys. Chem. Chem. Phys. 20 17387
 Google Scholar
Google Scholar
[18] Bian H, Duan H, Li J, Chen F, Cao B, Long M 2019 AIP Adv. 9 065207
 Google Scholar
Google Scholar
[19] Kundu S, Naik M H, Jain M 2020 Phy. Rev. Mater. 4 054004
 Google Scholar
Google Scholar
[20] Luo M, Xu Y E, Song Y X 2018 J. Supercond. Novel Magn. 31 449
 Google Scholar
Google Scholar
[21] Ding Y M, Shi J J, Zhang M, Zhu Y H, Wu M, Wang H, Cen Y L, Guo W H, Pan S H 2018 Physica E 101 245
 Google Scholar
Google Scholar
[22] Chen HL, Han J N, Deng X Q, Fan Z Q, Sun L, Zhang Z H 2022 Appl. Surf. Sci. 598 153756
 Google Scholar
Google Scholar
[23] Han J N, Zhang Z H, Fan Z Q, Zhou R L 2020 Nanotechnology 31 315206
 Google Scholar
Google Scholar
[24] Hu R, Wang D, Fan Z Q, Zhang Z H 2018 Phys. Chem. Chem. Phys. 20 13574
 Google Scholar
Google Scholar
[25] Chen H L, Zhang L, Deng X Q, Sun L, Zhang Z H, Fan Z Q 2021 J. Mater. Chem. C 9 12904
 Google Scholar
Google Scholar
[26] Zhao T, Fan Z Q, Zhang Z H, Zhou R L 2019 J. Phys. D: Appl. Phys. 52 475301
 Google Scholar
Google Scholar
[27] Hu R, Li Y H, Zhang Z H, Fan Z Q, Sun L 2019 J. Mater. Chem. C 7 7745
 Google Scholar
Google Scholar
[28] Dong Q X, Hu R, Fan Z Q, Zhang Z H 2018 Carbon 130 206
 Google Scholar
Google Scholar
[29] Hu J K, Zhang Z H, Fan Z Q, Zhou R L 2019 Nanotechnology 30 485703
 Google Scholar
Google Scholar
[30] Han J N, He X, Fan Z Q, Zhang Z H 2019 Phys. Chem. Chem. Phys. 21 1830
 Google Scholar
Google Scholar
[31] Grimme S 2006 J. Comput. Chem. 27 1787
 Google Scholar
Google Scholar
[32] Yang Y Y, Gong P, Ma W D, Hao R, Fang X Y 2021 Chin. Phys. B 30 067803
 Google Scholar
Google Scholar
[33] Jia Y H, Gong P, Li S L, Ma W D, Fang X Y, Yang Y Y 2020 Phys. Lett. A 384 126106
 Google Scholar
Google Scholar
[34] 吴甜, 姚梦丽, 龙孟秋 2021 70 056301
 Google Scholar
Google Scholar
Wu T, Yao M L, Long M Q 2021 Acta Phys. Sin. 70 056301
 Google Scholar
Google Scholar
[35] Gong P, Yang Y Y, Ma W D, Fang X Y, Jing X L, Jia Y H, Cao M S 2021 Physica E 128 114578
 Google Scholar
Google Scholar
[36] Xie Z J, Zhang B, Ge Y Q, Zhu Y, Nie G H, Song Y F, Lim C K, Zhang H, Prasad P N 2021 Chem. Rev. 122 1127
 Google Scholar
Google Scholar
[37] He X, Fan Z Q, Zhang Z H 2020 Phys. Chem. Chem. Phys. 22 23665
 Google Scholar
Google Scholar
-
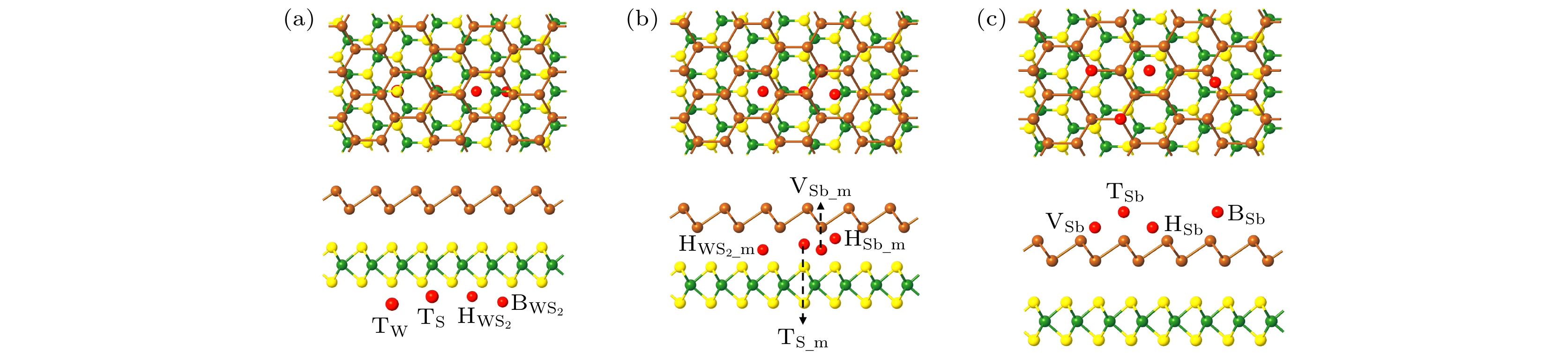
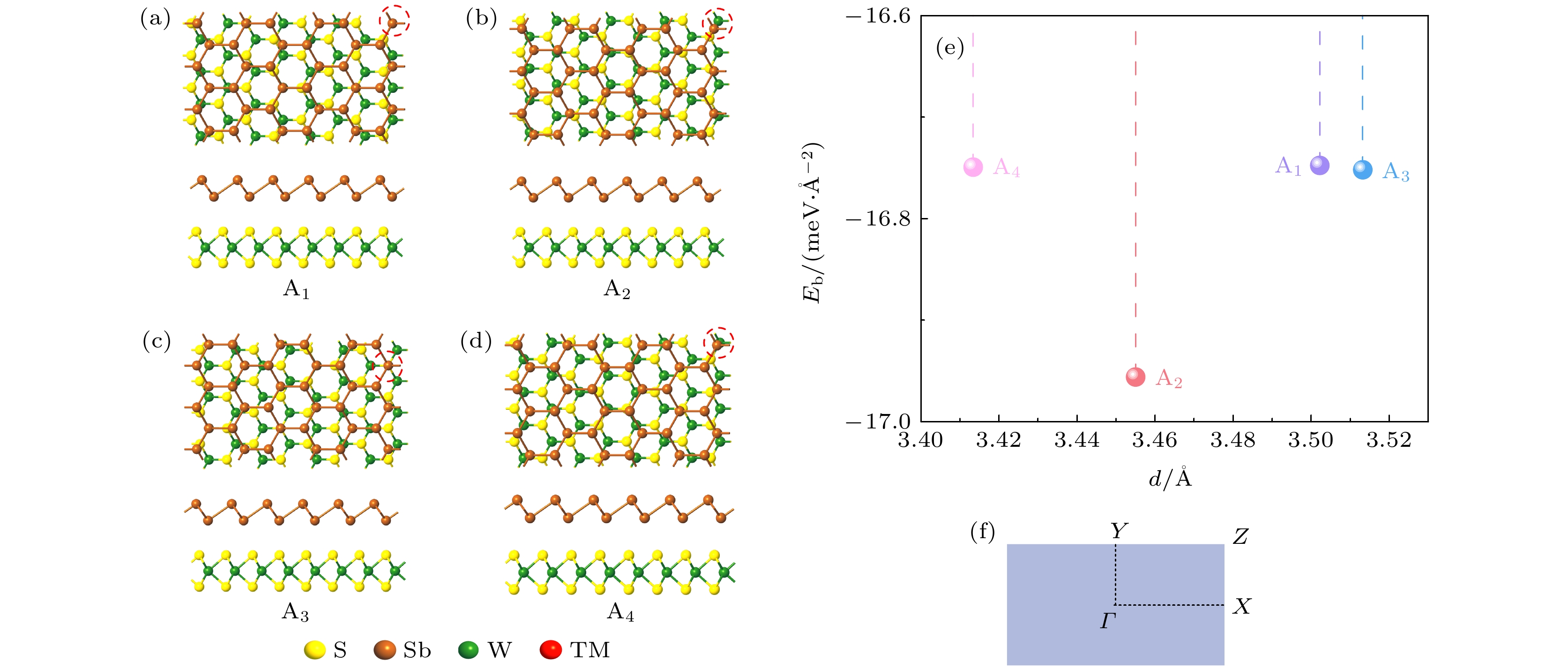
图 1 (a)—(d) 优化之后Sb/WS2异质结的四种堆垛方式的主视图和侧视图; (e) 优化后不同异质结的层间距与结合能; (f) 异质结单胞对应的布里渊区
Figure 1. (a)–(d) Top and side views of the four stacking patterns of optimized Sb/WS2 heterostructures; (e) the binding energy and interlayer distance of the optimized heterostructures; (f) Brillouin zone corresponding to heterostructure unit-cell.
图 2 PBE计算的(a) Sb单层、(b) WS2单层和(c) Sb/WS2异质结的能带结构; HSE06计算的(d) Sb单层、(e) WS2单层和(f) Sb/WS2异质结的能带结构
Figure 2. Band structures by PBE calculation: (a) Sb monolayer; (b) WS2 monolayer; (c) Sb/WS2 heterostructure. The band structures by HSE06 calculation: (d) Sb monolayer; (e) WS2 monolayer; (f) Sb/WS2 heterostructure.
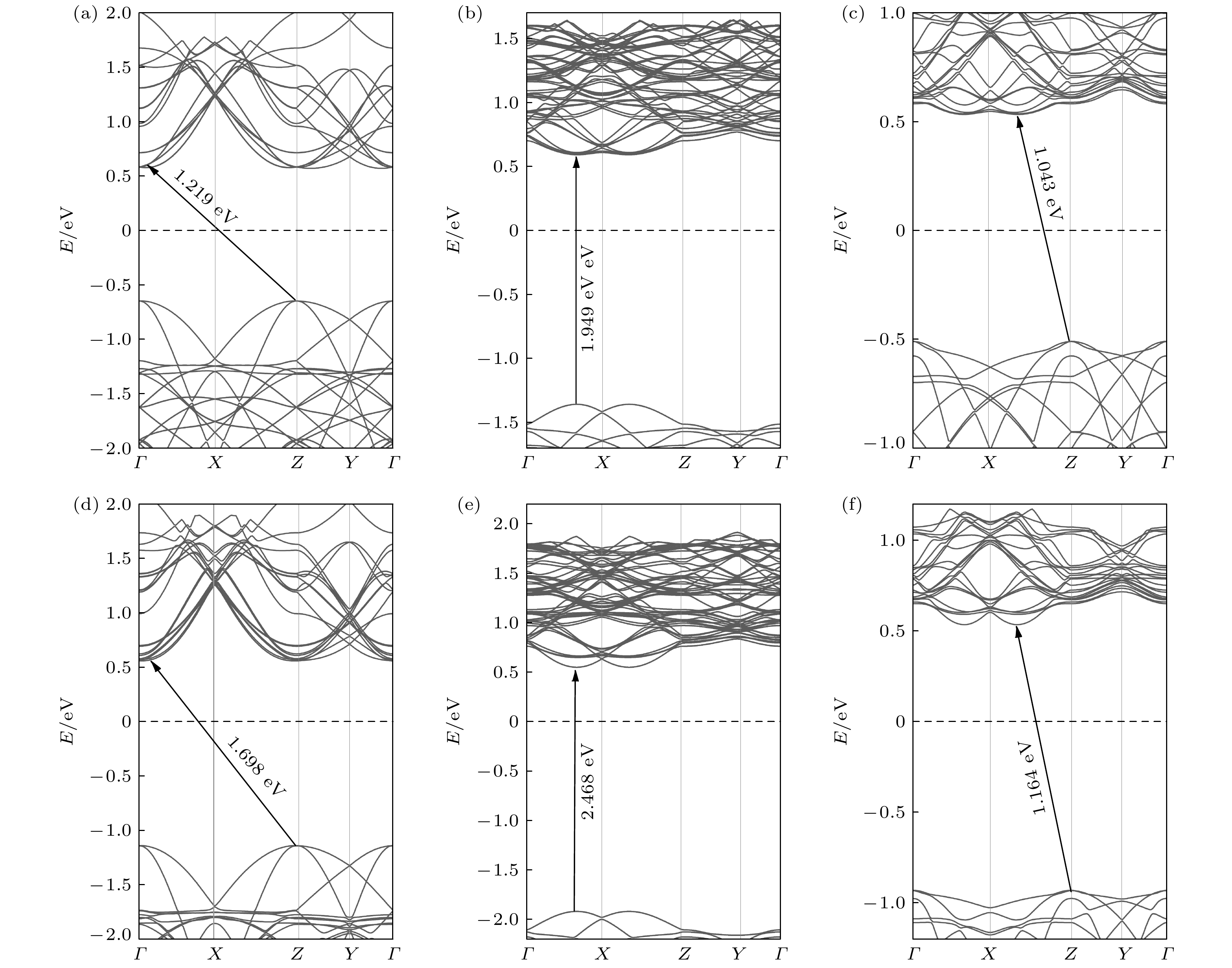
图 5 对应最稳三种吸附位置的异质结能带结构、态密度以及投影态密度 (a)—(c) 能带结构; (d)—(f) 态密度和投影态密度
Figure 5. Band structure, density of states and projected density of states of the heterostructures corresponding to three most stable adsorption sites: (a)–(c) Band structure; (d)–(f) density of states and projected density of states.
图 6 (a) 外加电场示意图; (b) 电场能、带隙值及磁性随加电场变化; (c)—(j) Eext = –0.1, –0.3, –0.7, –1, 0.2, 0.5, 0.8 和1.0 V/Å时的能带结构, 其中图(d)中绿色区域代表该阴影部分的能带放大图
Figure 6. (a) Schematic diagram of applied external electric field on heterostructure. (b) Electric field energy, band gap, and magnetic phase versus the external electric field. (c)–(j) The band structures for Eext = –0.1, –0.3, –0.7, –1, 0.2, 0.5, 0.8, and 1.0 V/Å, where the green region in panel (d) represents the enlarged partial band structure.
图 7 (a)施加的拉力与压力示意图; (b) 应变能、带隙及磁相随应变变化; (c)—(f) ε = –0.3, 0.2, 0.35 和 0.4 Å时的能带结构, 其中图(e)与图(f)中绿色区域代表该部分的能带放大图
Figure 7. (a) Schematic diagram of stretching and compressing heterostructure; (b) the strain energy, band gap and magnetic phase as versus strain; (c)–(f) band structure at ε = –0.3, 0.2, 0.35, and 0.4 Å, where the green region in panel (e) and (f) represent the enlarged partial band structure.
表 1 磁矩、TM原子电子构型、电荷转移和磁化能. μ0 为孤立Fe原子的磁矩, μ为Fe原子吸附后磁矩, 括号中M为超元胞磁矩. VEC 为孤立Fe原子价电子构型(valence electron configuration), VEC*为Fe原子吸附后价电子构型. ΔQ为吸附之后Fe原子所转移的电荷, “–”代表 Fe原子失去电荷. EM为磁化能
Table 1. Magnetic moment, electron configuration of TM atom, charge transfer and magnetization energy. μ0 is the magnetic moment of isolated Fe atoms, μ is the magnetic moment after adsorption of Fe atoms, M in parentheses is the magnetic moment of the supercell. VEC is the valence electron configuration of isolated Fe atoms, and VEC* is the valence electron configuration of Fe atoms after adsorption. ΔQ is the charge transferred by Fe atom after adsorption, where “–” means that the Fe atom loses its charge. EM is the magnetization energy.
Adsorbed site μ0/μB μ/μB (M/μB) VEC VEC* ΔQ/|e| EM/meV TW 4 2.002 (2.023) 3d/4s
6/23d/4s/4p
6.923/0.354/0.304–0.423 75.3 TS_m 4 2.002 (2.290) 3d/4s
6/23d/4s/4p
7.011/0.568/0.304–0.119 146.46 VSb 4 2.004 (2.313) 3d/4s
6/23d/4s/4p
7.030/0.577/0.330–0.065 10.02 -
[1] Geim A K, Grigorieva I V 2013 Nature 499 419
 Google Scholar
Google Scholar
[2] Li X M, Tao L, Chen Z F, Fang H, Li X S, Wang X R, Xu J B, Zhu H W 2017 Appl. Phys. Rev. 4 021306
 Google Scholar
Google Scholar
[3] Novoselov K S, Mishchenko A, Carvalho A, Neto A H C 2016 Science 353 aac9439
 Google Scholar
Google Scholar
[4] Shang J M, Pan L F, Wang X T, Li J B, Deng H X, Wei Z M 2018 J. Mater. Chem. C 6 7201
 Google Scholar
Google Scholar
[5] Idrees M, Fawad M, Bilal M, Saeed Y, Nguyen C, Amin B 2020 RSC Adv. 10 25801
 Google Scholar
Google Scholar
[6] Ozcelik V O, Azadani J G, Yang C, Koester S J, Low T 2016 Phys. Rev. B 94 035125
 Google Scholar
Google Scholar
[7] Liu C H, Clark G, Fryett T, Wu S F, Zheng J J, Hatami F, Xu X D, Majumdar A 2017 Nano Lett. 17 200
 Google Scholar
Google Scholar
[8] Binder J, Withers F, Molas M R, Faugeras C, Nogajewski K, Watanabe K, Taniguchi T, Kozikov A, Geim A K, Novoselov K S, Potemski M 2017 Nano Lett. 17 1425
 Google Scholar
Google Scholar
[9] He X, Deng X Q, Sun L, Zhang Z H, Fan Z Q 2022 Appl. Surf. Sci. 578 151844
 Google Scholar
Google Scholar
[10] Huang L, Huo N J, Li Y, Chen H, Yang J H, Wei Z M, Li J B, Li S S 2015 J. Phys. Chem. Lett. 6 2483
 Google Scholar
Google Scholar
[11] Xiao W Z, Xu L, Rong Q Y, Dai X Y, Cheng C P, Wang L L 2020 Appl. Surf. Sci. 504 144425
 Google Scholar
Google Scholar
[12] Huang L, Li Y, Wei Z M, Li J B 2015 Sci. Rep. 5 16448
 Google Scholar
Google Scholar
[13] Lei C G, Ma Y D, Xu X L, Zhang T, Huang B B, Dai Y 2019 J. Phys. Chem. C 123 23089
 Google Scholar
Google Scholar
[14] Yan R S, Fathipour S, Han Y M, Song B, Xiao S D, Li M D, Ma N, Protasenko V, Muller D A, Jena D, Xing H G 2015 Nano Lett. 15 5791
 Google Scholar
Google Scholar
[15] Shim J, Oh S, Kang D H, Jo S H, Ali M H, Choi W Y, Heo K, Jeon J, Lee S, Kim M, Song Y J, Park J H 2016 Nat. Commun. 7 13413
 Google Scholar
Google Scholar
[16] Xia C X, Du J, Li M, Li X P, Zhao X, Wang T X, Li J B 2018 Phy. Rev. A 10 054064
 Google Scholar
Google Scholar
[17] Wu Y B, Huang Z Y, Liu H T, He C Y, Xue L, Qi X, Zhong J X 2018 Phys. Chem. Chem. Phys. 20 17387
 Google Scholar
Google Scholar
[18] Bian H, Duan H, Li J, Chen F, Cao B, Long M 2019 AIP Adv. 9 065207
 Google Scholar
Google Scholar
[19] Kundu S, Naik M H, Jain M 2020 Phy. Rev. Mater. 4 054004
 Google Scholar
Google Scholar
[20] Luo M, Xu Y E, Song Y X 2018 J. Supercond. Novel Magn. 31 449
 Google Scholar
Google Scholar
[21] Ding Y M, Shi J J, Zhang M, Zhu Y H, Wu M, Wang H, Cen Y L, Guo W H, Pan S H 2018 Physica E 101 245
 Google Scholar
Google Scholar
[22] Chen HL, Han J N, Deng X Q, Fan Z Q, Sun L, Zhang Z H 2022 Appl. Surf. Sci. 598 153756
 Google Scholar
Google Scholar
[23] Han J N, Zhang Z H, Fan Z Q, Zhou R L 2020 Nanotechnology 31 315206
 Google Scholar
Google Scholar
[24] Hu R, Wang D, Fan Z Q, Zhang Z H 2018 Phys. Chem. Chem. Phys. 20 13574
 Google Scholar
Google Scholar
[25] Chen H L, Zhang L, Deng X Q, Sun L, Zhang Z H, Fan Z Q 2021 J. Mater. Chem. C 9 12904
 Google Scholar
Google Scholar
[26] Zhao T, Fan Z Q, Zhang Z H, Zhou R L 2019 J. Phys. D: Appl. Phys. 52 475301
 Google Scholar
Google Scholar
[27] Hu R, Li Y H, Zhang Z H, Fan Z Q, Sun L 2019 J. Mater. Chem. C 7 7745
 Google Scholar
Google Scholar
[28] Dong Q X, Hu R, Fan Z Q, Zhang Z H 2018 Carbon 130 206
 Google Scholar
Google Scholar
[29] Hu J K, Zhang Z H, Fan Z Q, Zhou R L 2019 Nanotechnology 30 485703
 Google Scholar
Google Scholar
[30] Han J N, He X, Fan Z Q, Zhang Z H 2019 Phys. Chem. Chem. Phys. 21 1830
 Google Scholar
Google Scholar
[31] Grimme S 2006 J. Comput. Chem. 27 1787
 Google Scholar
Google Scholar
[32] Yang Y Y, Gong P, Ma W D, Hao R, Fang X Y 2021 Chin. Phys. B 30 067803
 Google Scholar
Google Scholar
[33] Jia Y H, Gong P, Li S L, Ma W D, Fang X Y, Yang Y Y 2020 Phys. Lett. A 384 126106
 Google Scholar
Google Scholar
[34] 吴甜, 姚梦丽, 龙孟秋 2021 70 056301
 Google Scholar
Google Scholar
Wu T, Yao M L, Long M Q 2021 Acta Phys. Sin. 70 056301
 Google Scholar
Google Scholar
[35] Gong P, Yang Y Y, Ma W D, Fang X Y, Jing X L, Jia Y H, Cao M S 2021 Physica E 128 114578
 Google Scholar
Google Scholar
[36] Xie Z J, Zhang B, Ge Y Q, Zhu Y, Nie G H, Song Y F, Lim C K, Zhang H, Prasad P N 2021 Chem. Rev. 122 1127
 Google Scholar
Google Scholar
[37] He X, Fan Z Q, Zhang Z H 2020 Phys. Chem. Chem. Phys. 22 23665
 Google Scholar
Google Scholar
Catalog
Metrics
- Abstract views: 4734
- PDF Downloads: 61
- Cited By: 0














 DownLoad:
DownLoad: