-
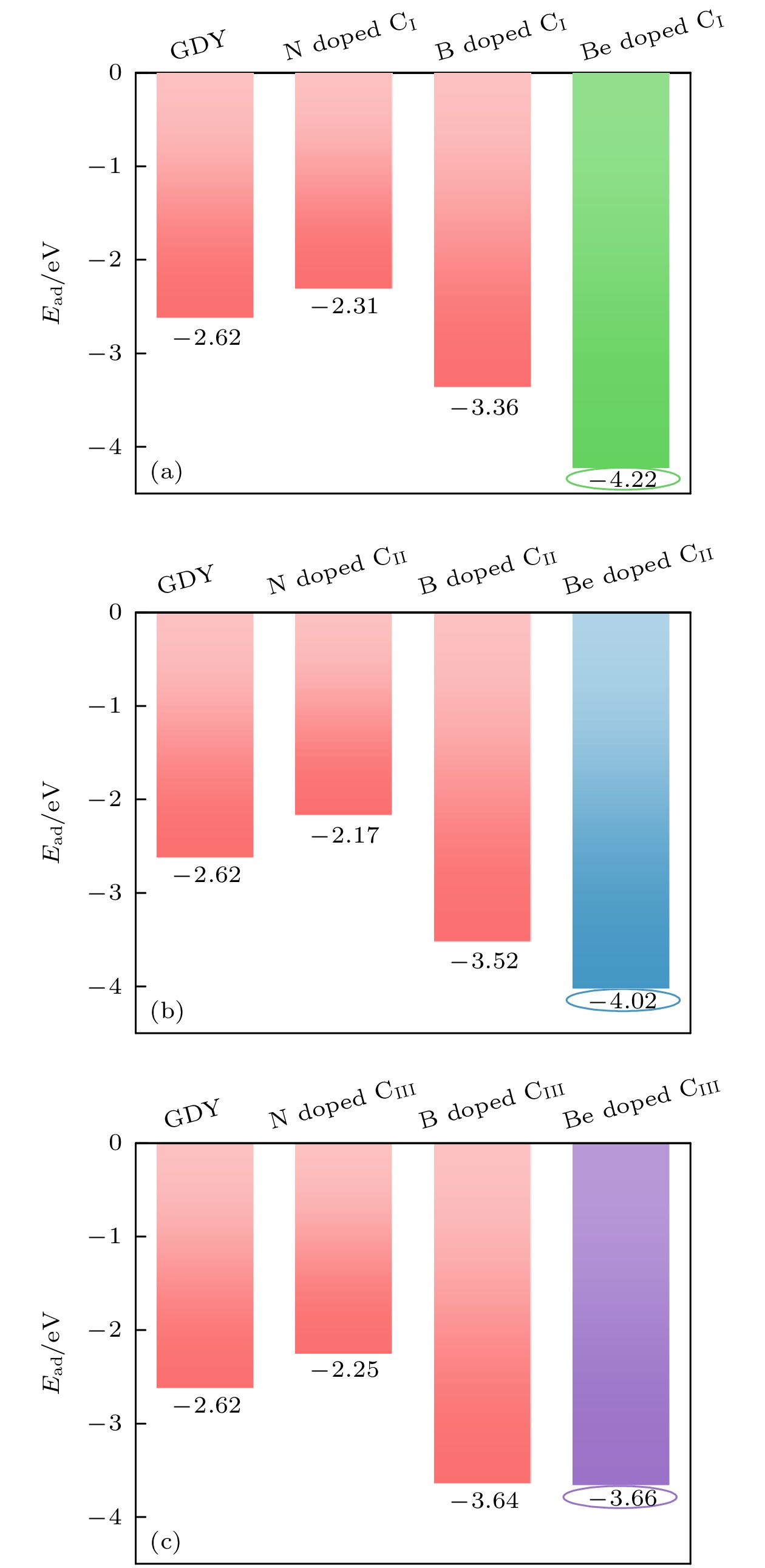
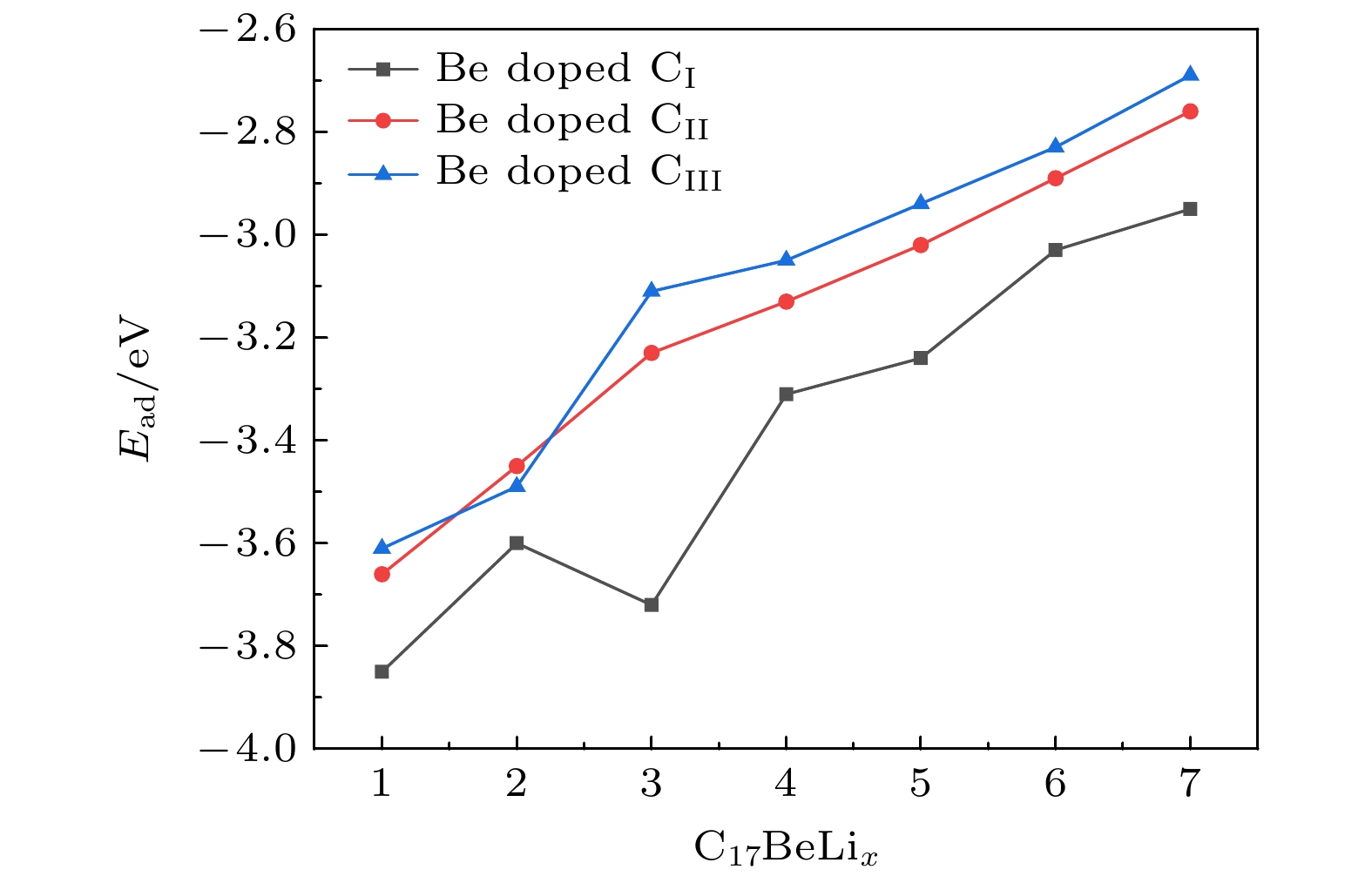
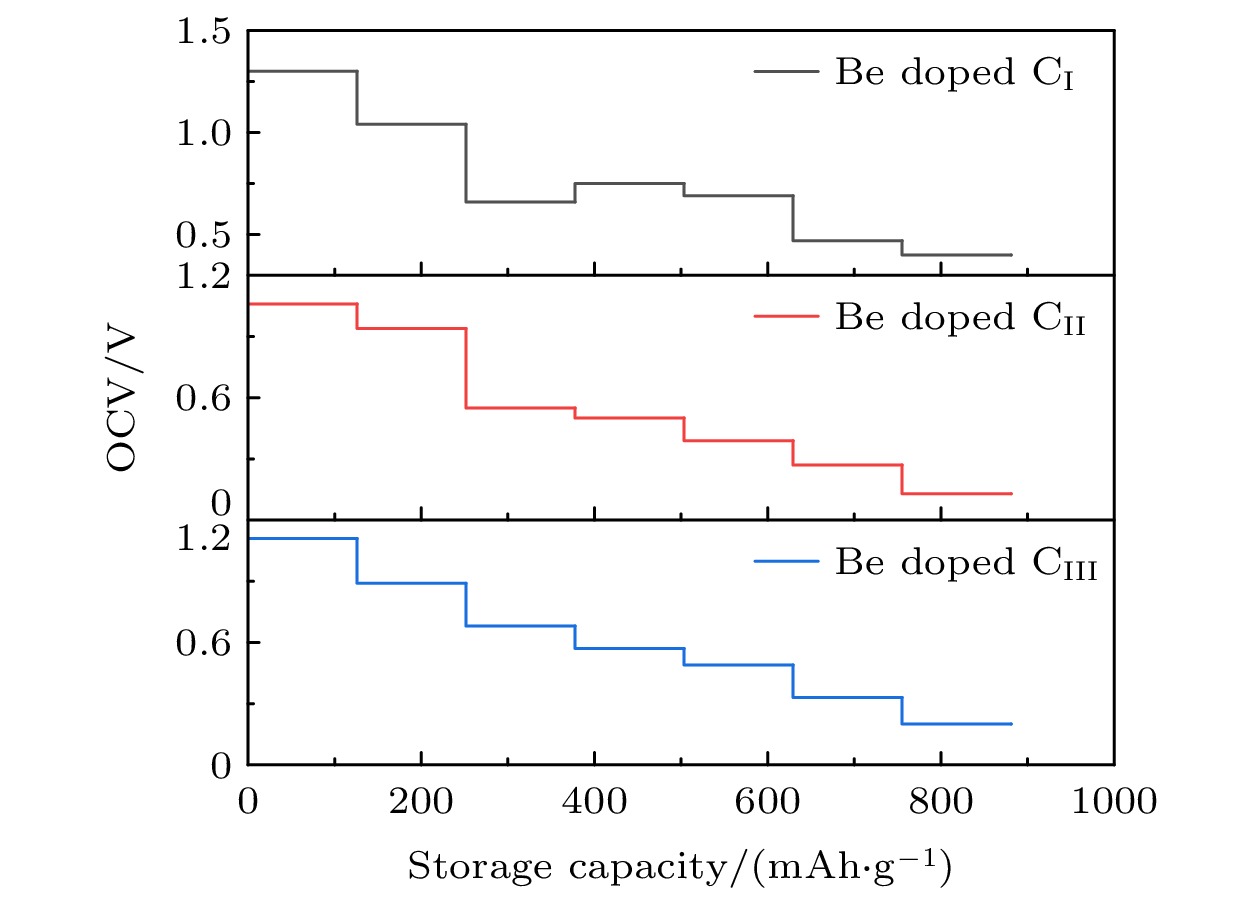
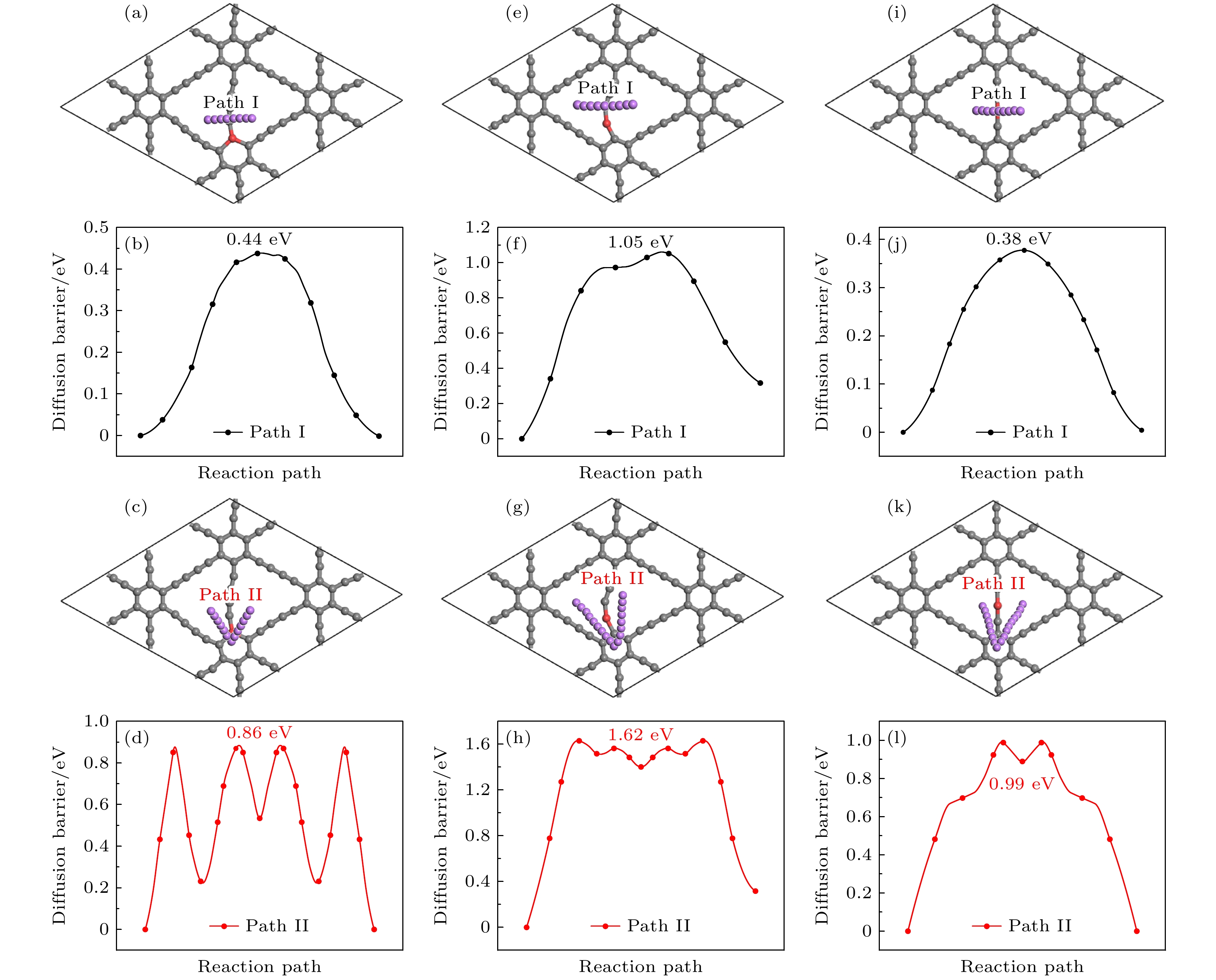
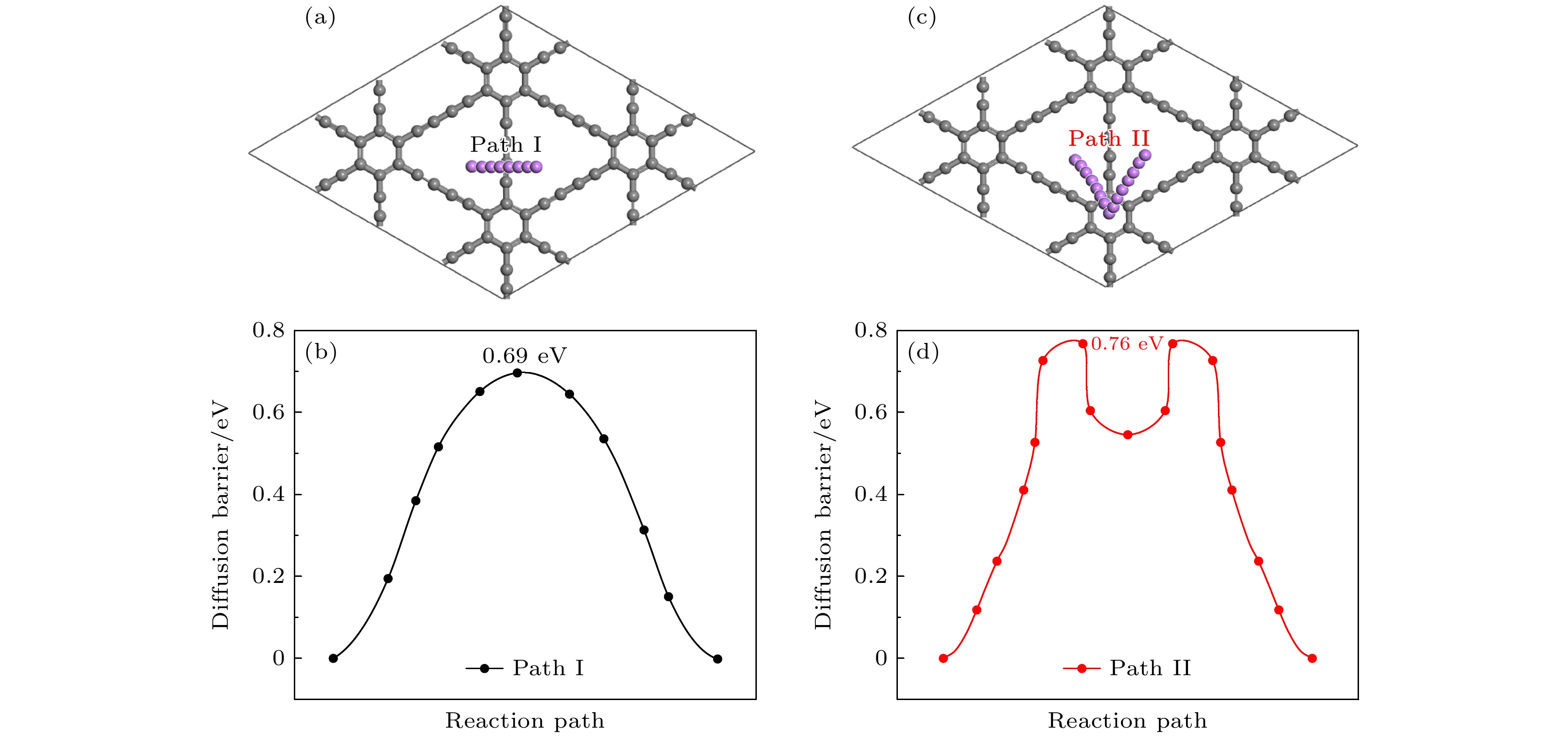
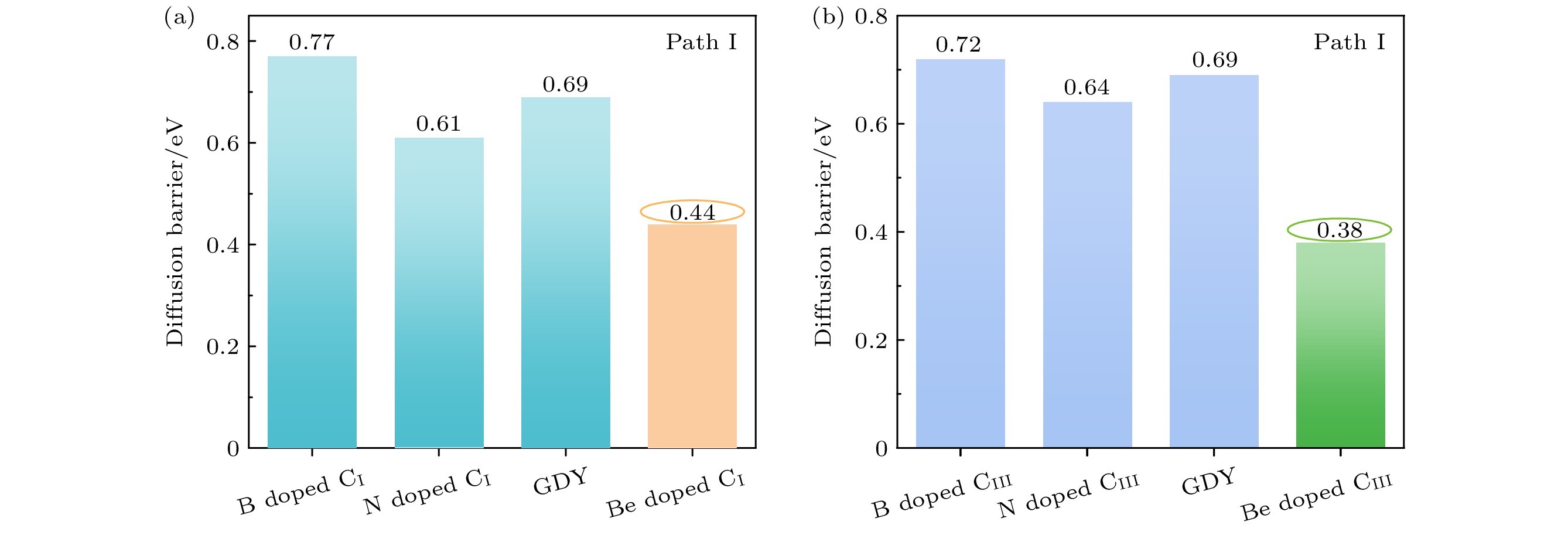
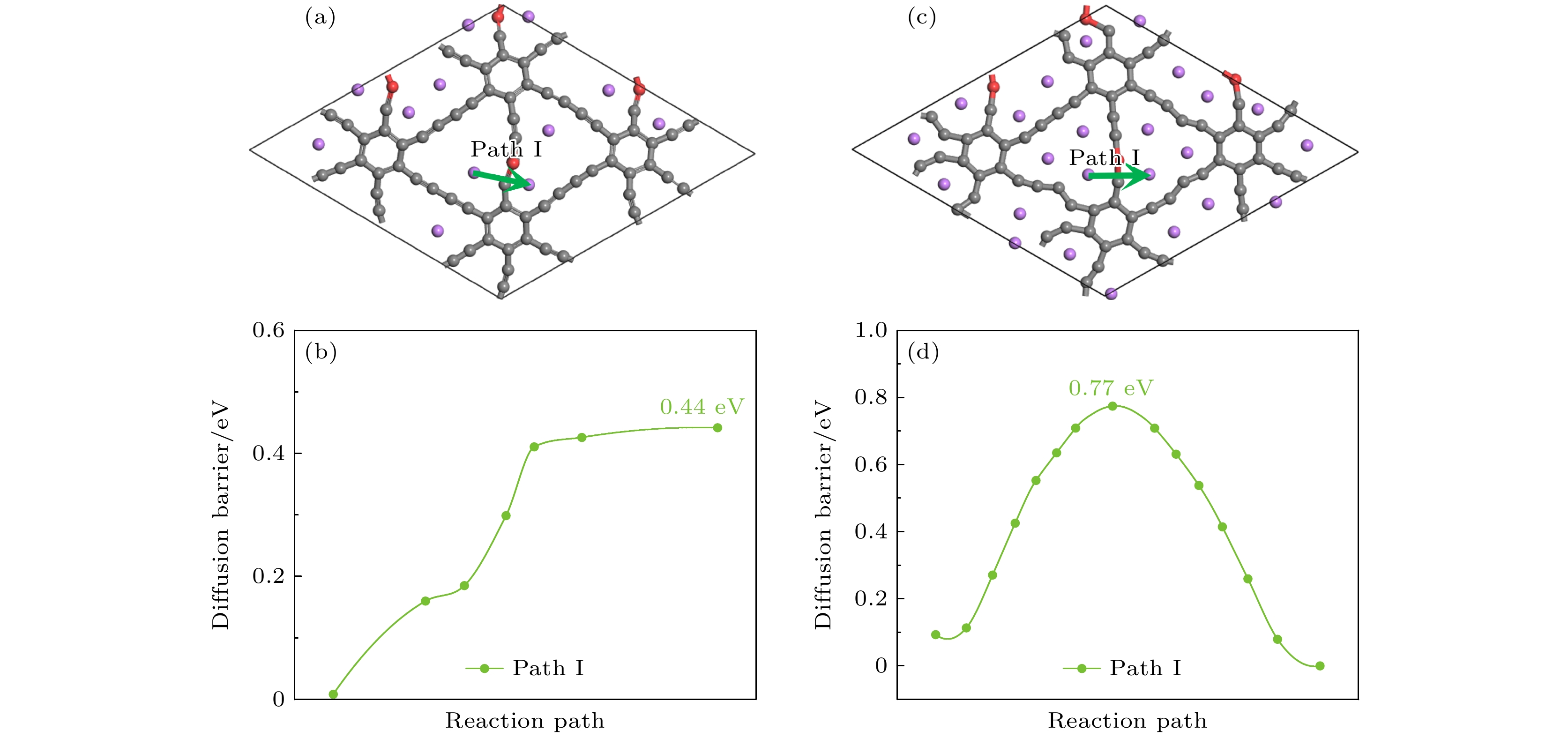
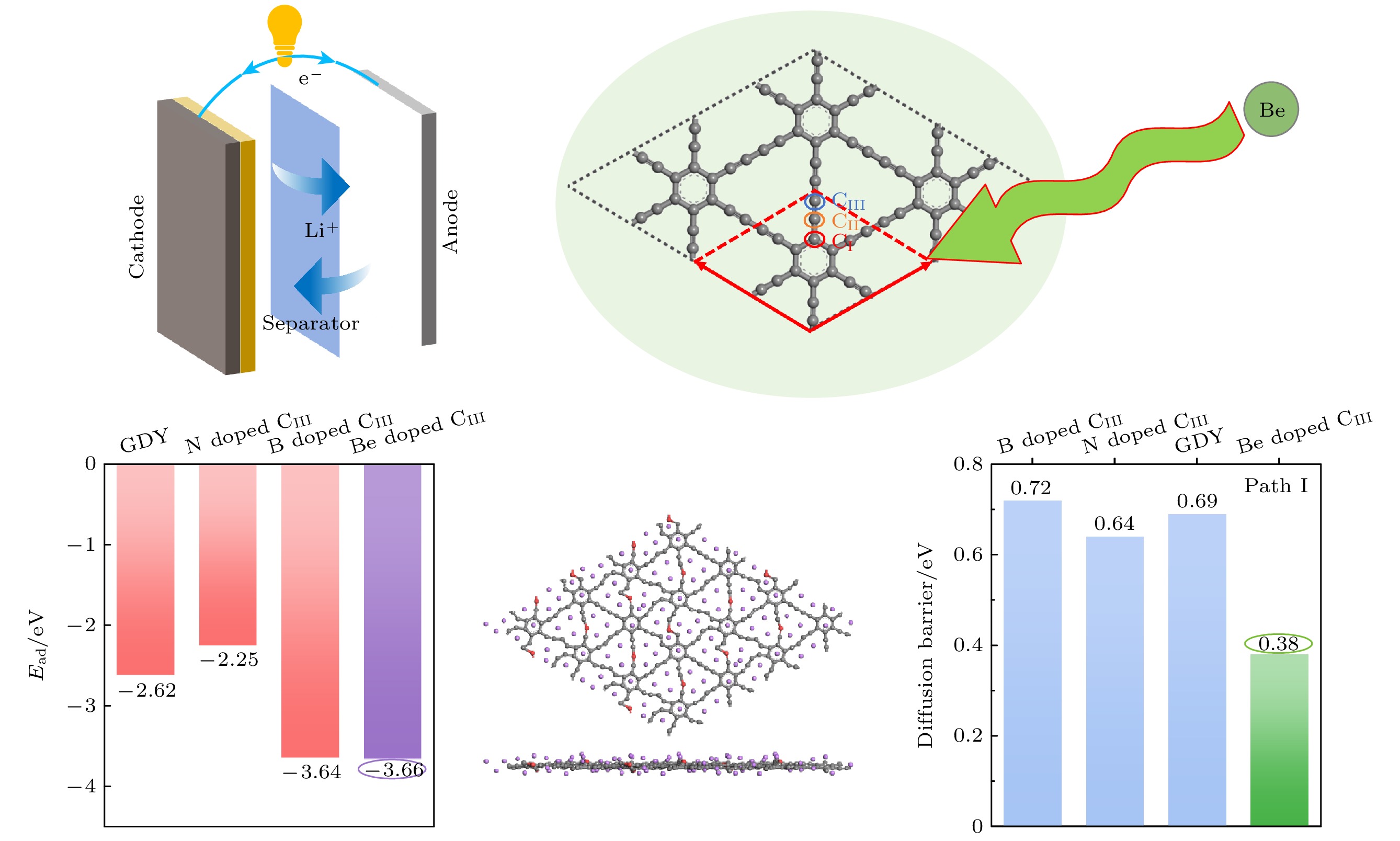
采用基于密度泛函理论的第一性原理方法, 研究了Be在不同位置掺杂的石墨双炔作为锂离子电池负极材料的性能. 通过计算不同掺杂浓度下石墨双炔的形成能和内聚能, 表明Be掺杂的石墨双炔具有很好的实验合成前景. 更重要的是, 掺Be后的石墨双炔具有良好的导电性能. Be掺杂的石墨双炔对单个锂的吸附能为–4.22 eV, 相较于硼, 氮掺杂石墨双炔以及本征石墨双炔大幅度提升. 随着储锂数量的增加, 其对锂的吸附能依旧大于固体锂的内聚能, 并且平均开路电压在0—1 V之间, 充分保证了电池的安全性. 除此之外, 将储锂容量提升为881 mAh/g, 是未掺杂石墨双炔的1.14倍, 是石墨的2.36倍. 同时, 锂在Be掺杂的石墨双炔上扩散性能也有所提升. 对于CIII位掺杂体系, 通过研究低锂浓度、中锂浓度、高锂浓度阶段的离子输运. 研究发现随着锂浓度的增大, 扩散势垒分别为0.38, 0.44, 0.77 eV, 锂离子的移动变得困难, 但依然优于其他元素掺杂的石墨双炔. 综上所述, 铍掺杂石墨双炔作为优异的锂离子电池负极材料具有很大潜力.The performances of beryllium-doped graphdiyne (GDY), which is used as an anode material for lithium-ion batteries at various doping sites, are investigated by first-principles methods based on density functional theory. Calculations of the formation energy and cohesive energy of GDY at different doping concentrations indicate that beryllium-doped GDY has excellent prospects for experimental synthesis. More importantly, the beryllium-doped GDY exhibits good electrical conductivity. The adsorption energy for a single lithium atom on beryllium-doped GDY is –4.22 eV, which is significantly higher than that for boron, nitrogen-doped GDY, and intrinsic GDY. As the number of stored lithium atoms increases, the adsorption energy remains greater than the cohesive energy of solid lithium, and the average open-circuit voltage stays between 0 and 1 V, ensuring the safety of the battery. Additionally, the lithium storage capacity is increased to 881 mAh/g, which is 1.14 times that of undoped GDY and 2.36 times that of graphite. Meanwhile, the diffusion performance of lithium on beryllium-doped GDY is also enhanced. For the CIII site doping system, by studying the ion transports at low, medium, and high lithium concentrations, we find that as the lithium concentration increases, the diffusion barriers are 0.38, 0.44, and 0.77 eV, respectively, making lithium ion movement more difficult, but still superior to those of other element-doped GDY. In summary, beryllium-doped GDY has great potential as an excellent anode material for lithium-ion batteries.
-
Keywords:
- graphdiyne /
- lithium-ion batteries /
- density functional theory
[1] Erickson E M, Ghanty C, Aurbach D 2014 J. Phys. Chem. Lett. 5 3313
 Google Scholar
Google Scholar
[2] Novoselov K S, Geim A K, Morozov S V, Jiang D, Zhang Y, Dubonos S V, Grigorieva I V, Firsov A A 2004 Science 306 666
 Google Scholar
Google Scholar
[3] Yoo E, Kim J, Hosono E, Zhou H S, Kudo T, Honma I 2008 Nano Lett. 8 2277
 Google Scholar
Google Scholar
[4] Liu X, Wang C Z, Yao Y X, Lu W C, Hupalo M, Tringides M C, Ho K M 2011 Phys. Rev. B 83 235411
 Google Scholar
Google Scholar
[5] Li W, He Y H, Wang L, Ding G H, Zhang Z Q, Lortz R W, Sheng P, Wang N 2011 Phys. Rev. B 84 045431.
 Google Scholar
Google Scholar
[6] Liu X, Hupalo M, Wang C Z, Lu W C, Tringides M C 2012 Phys. Rev. B 86 081414
 Google Scholar
Google Scholar
[7] Baughman R H, Eckhardt H, Kertesz M 1987 J. Chem. Phys. 87 6687
 Google Scholar
Google Scholar
[8] Li G X, Li Y L, Liu H B, Guo Y B, Li Y J, Zhu D B 2010 Chem. Commun. 46 3256
 Google Scholar
Google Scholar
[9] Narita N, Nagai S, Suzuki S, Nakao K 1998 Phys. Rev. B 58 11009
 Google Scholar
Google Scholar
[10] Zhang H Y, Xia Y Y, Bu H X, Wang X P, Zhang M, Zhao L X, Luo T H, Zhao M W 2013 J. Appl. Phys. 113 044309
 Google Scholar
Google Scholar
[11] He J J, Wang N, Cui Z L, Du H P, Fu L, Huang C S, Yang Z, Shen X Y, Yi Y P, Tu Z Y, Li Y L 2017 Nat. Commun. 8 1172
 Google Scholar
Google Scholar
[12] Urbain F, Smirnov V, Becker J P, et al. 2016 Energ. Environ. Sci. 9 145
 Google Scholar
Google Scholar
[13] Wang N, He J J, Tu Z Y, Yang Z, Zhao F H, Li X D, Huang C D, Wang K, Jiu T G, Yi Y P, Li Y L 2017 Angew. Chem. Int. Edit. 56 10740
 Google Scholar
Google Scholar
[14] Shen X Y, Li X D, Zhao F H, Wang N, Xie C P, He J J, Si W Y, Yi Y Y, Yang Z, Li X F, Lu F S, Huang C S 2019 2D Materials 6 035020
 Google Scholar
Google Scholar
[15] 曾雯2020 硕士学位论文 (重庆: 重庆大学)
Zeng W 2020 M. S. Thesis (Chongqing: Chongqing University
[16] Wang N, Li X D, Tu Z Y, Zhao F H, He J J, Guan Z Y, Huang C D, Yi Y P, Li Y L 2018 Angew. Chem. Int. Edit. 57 3968
 Google Scholar
Google Scholar
[17] Yang Z, Liu R R, Wang N, He J J, Wang K, Li X D, Shen X Y, Wang X, Lv Q, Zhang M J, Luo J, Jiu T G, Hou Z F, Huang C S 2018 Carbon 137 442
 Google Scholar
Google Scholar
[18] Yang Z, Shen X Y, Wang N, He J J, Li X D, Wang X, Hou Z F, Wang K, Gao J, Jiu T G, Huang C S 2019 ACS Appl. Mater. Interfaces 11 2608
 Google Scholar
Google Scholar
[19] Hussain A, Ullah S, Farhan M A 2016 RSC Adv. 6 55990
 Google Scholar
Google Scholar
[20] Ullah S, Hussain A, Syed W, Saqlain M A, Ahmad I, Leenaertse O, Karimf A 2015 RSC Adv. 5 55762
 Google Scholar
Google Scholar
[21] Kost F, Linsmeier Ch, Oberkofler M, Reinelt M, Balden M, Herrmann A, Lindig S 2009 J. Nucl. Mater. 390–391 975
 Google Scholar
Google Scholar
[22] Goldstraß P, Linsmeier C 2000 Nucl. Instrum. Meth. B 161–163 411
 Google Scholar
Google Scholar
[23] Anghel A, Porosnicu C, Lungu C P, Sugiyama K, Krieger C, Roth J 2011 J. Nucl. Mater. 416 9
 Google Scholar
Google Scholar
[24] Ferro Y, Allouche A, Linsmeier C 2013 J. Appl. Phys. 113 213514
 Google Scholar
Google Scholar
[25] Campbell A, Cakmak E, Henry B, et al. 2023 Be 2C Synthesis, Properties, and Ion-beam Irradiation Damage Characterization ORNL/TM-2023/3011
[26] López-Urías F, Terrones M, Terrones H 2015 Carbon 84 317
 Google Scholar
Google Scholar
[27] Ullah S, Denis P A, Sato F 2017 Appl. Mater. Today 9 333
 Google Scholar
Google Scholar
[28] Becke A D 1988 Phys. Rev. A 38 3098
 Google Scholar
Google Scholar
[29] Delley B 1990 J. Chem. Phys. 92 508
 Google Scholar
Google Scholar
[30] Perdew J P, Burke K, Ernzerhof M 1996 Phys. Rev. Lett. 77 3865
 Google Scholar
Google Scholar
[31] Long M Q, Tang L, Wang D, Li Y L, Shuai Z G 2011 ACS Nano 5 2593
 Google Scholar
Google Scholar
[32] Sun C, Searles D J 2012 J. Phys. Chem. C 116 26222
 Google Scholar
Google Scholar
[33] Hwang H J, Koo J, Park M, Park N, Kwon Y, Lee H 2013 J. Phys. Chem. C 117 6919
 Google Scholar
Google Scholar
[34] Zheng F C, Yang Y, Chen Q W 2014 Nat. Commun. 5 5261
 Google Scholar
Google Scholar
[35] Eftekhari A 2017 Energy Storage Mater. 7 157
 Google Scholar
Google Scholar
[36] 蔡梦圆, 唐春梅, 张秋月 2019 68 213601
 Google Scholar
Google Scholar
Cai M Y, Tang C M, Zhang Q Y 2019 Acta Phys. Sin. 68 213601
 Google Scholar
Google Scholar
[37] Jang B, Koo J, Park M, Lee H, Nam J, Kwon Y, Lee H 2013 Appl. Phys. Lett. 103 263904
 Google Scholar
Google Scholar
-
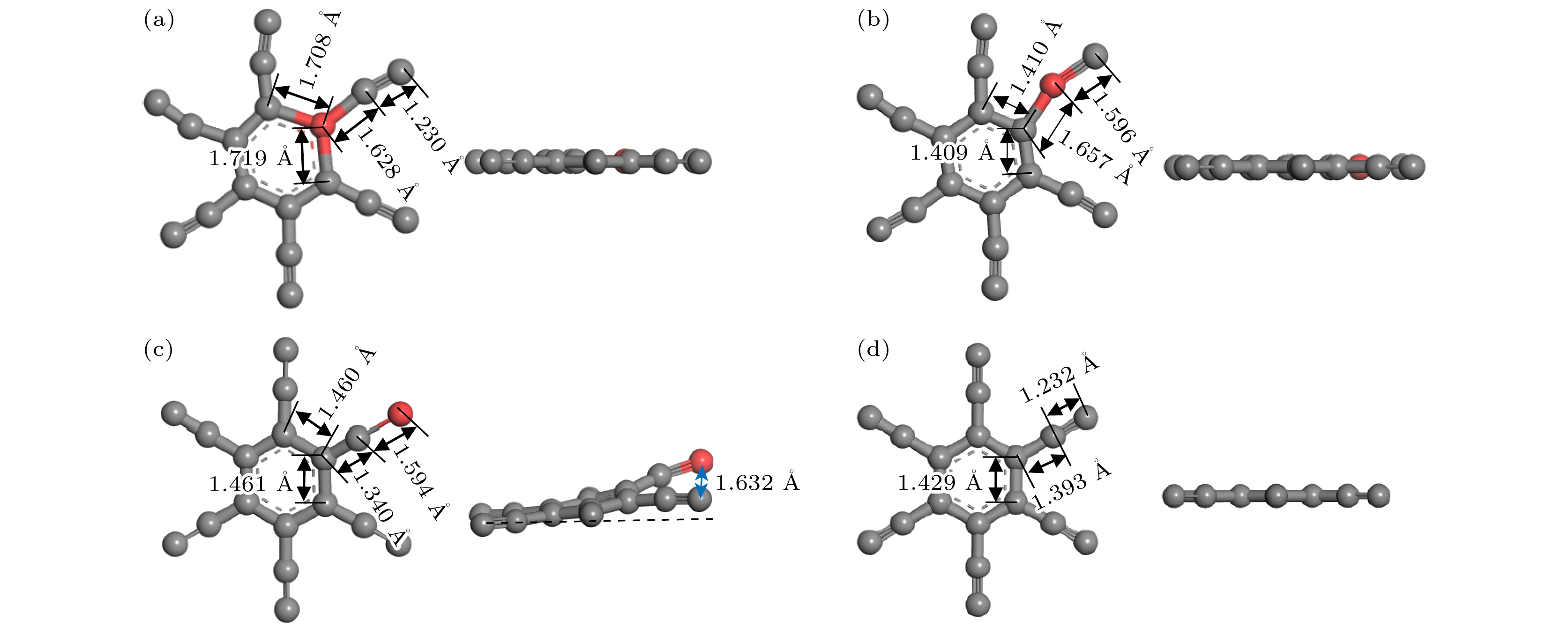
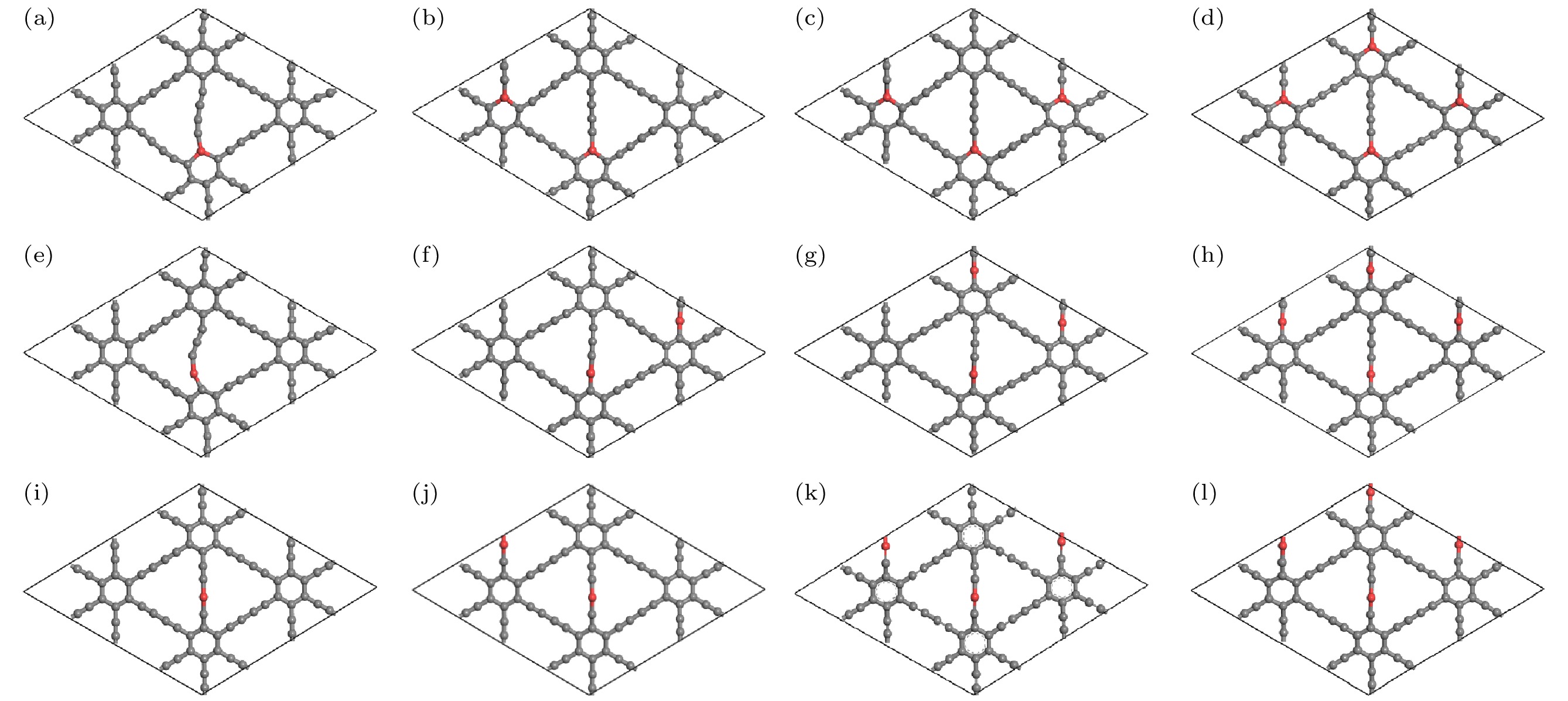
图 3 掺杂浓度分别为(a) 1.39%, (b) 2.78%, (c) 4.17%, (d) 5.56%的CI位掺杂体系弛豫后的几何结构; 掺杂浓度分别为(e) 1.39%, (f) 2.78%, (g) 4.17%, (h) 5.56%的CII位掺杂体系弛豫后的几何结构; 掺杂浓度分别为(i) 1.39%, (g) 2.78%, (k) 4.17%, (l) 5.56%的CIII位掺杂体系弛豫后的几何结构
Fig. 3. Relaxed geometric structures of the CI site doping systems with doping concentrations of (a) 1.39%, (b) 2.78%, (c) 4.17%, (d) 5.56%; relaxed geometric structures of the CII site doping systems with doping concentrations of (e) 1.39%, (f) 2.78%, (g) 4.17%, (h) 5.56%; relaxed geometric structures of the CIII site doping systems with doping concentrations of (i) 1.39%, (g) 2.78%, (k) 4.17%, (l) 5.56%.
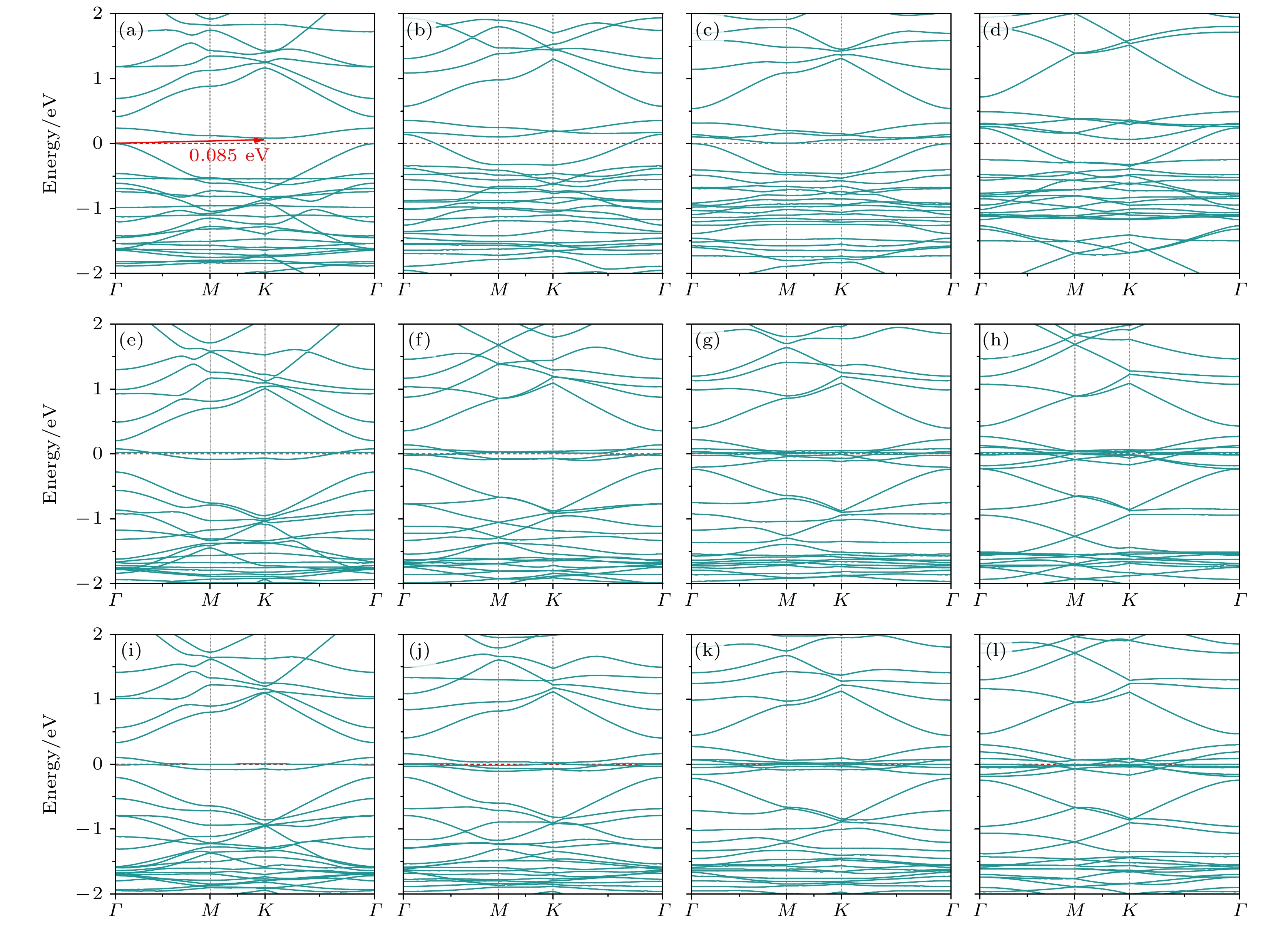
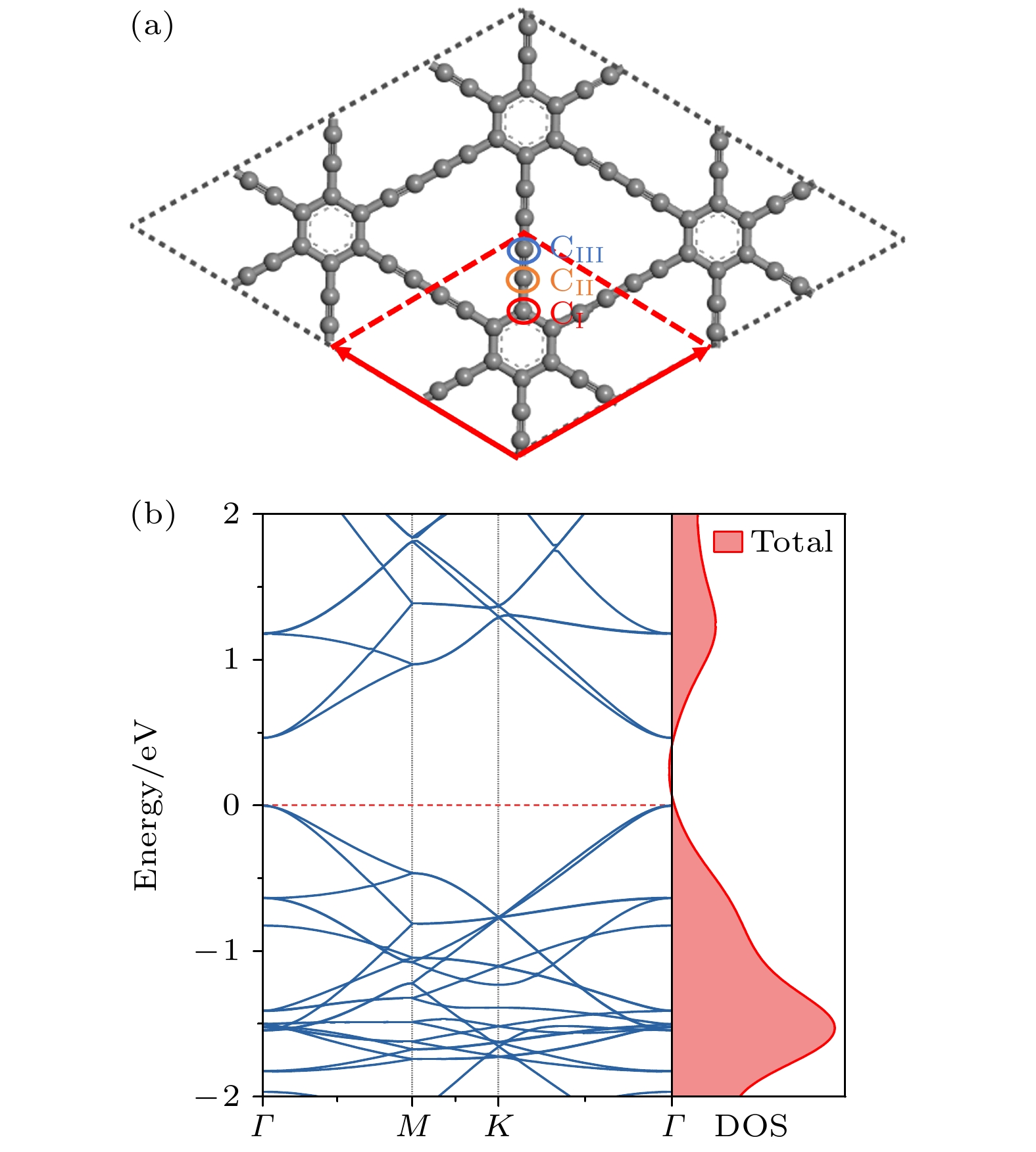
图 6 掺杂浓度分别为(a) 1.39%, (b) 2.78%, (c) 4.17%, (d) 5.56%的CI位替换掺杂的能带结构; 掺杂浓度分别为(e) 1.39%, (f) 2.78%, (g) 4.17%, (h) 5.56%的CII位替换掺杂的能带结构; 掺杂浓度分别为(i) 1.39%, (g) 2.78%, (k) 4.17%, (l) 5.56%的CIII位替换掺杂的能带结构
Fig. 6. Band structure of CI site replacement doping with doping concentrations of (a) 1.39%, (b) 2.78%, (c) 4.17%, (d) 5.56%; the band structure of CII site replacement doping with doping concentrations of (e) 1.39%, (f) 2.78%, (g) 4.17%, (h) 5.56%; the band structure of CIII site replacement doping with doping concentrations of (i) 1.39%, (g) 2.78%, (k) 4.17%, (l) 5.56%.
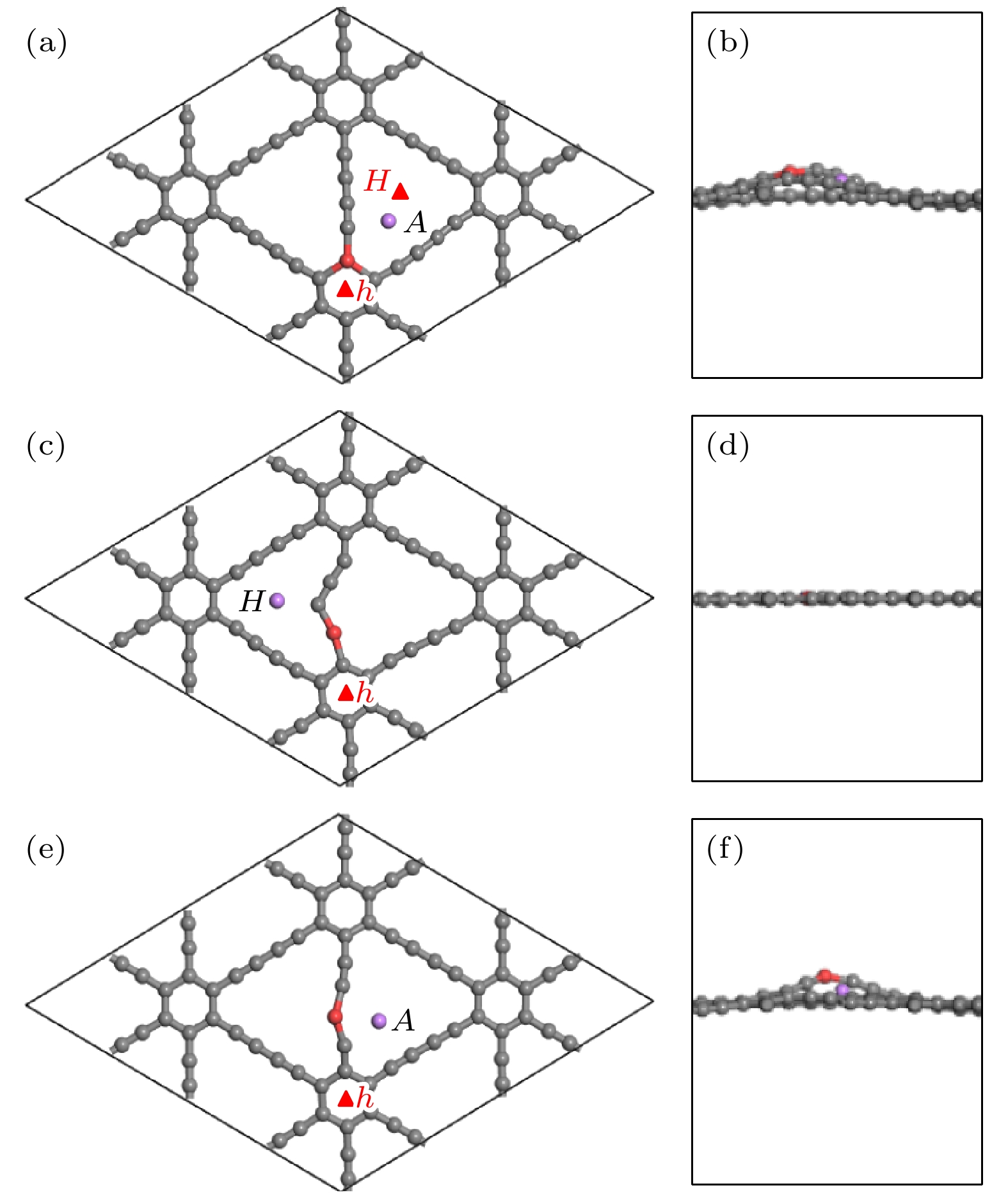
图 8 单个Li在CI位掺杂的稳定吸附位的(a) 俯视图和(b) 侧视图; 在CII位掺杂的稳定吸附位的(c) 俯视图和(d) 侧视图; 在CIII位掺杂的稳定吸附位的(e) 俯视图和(f) 侧视图
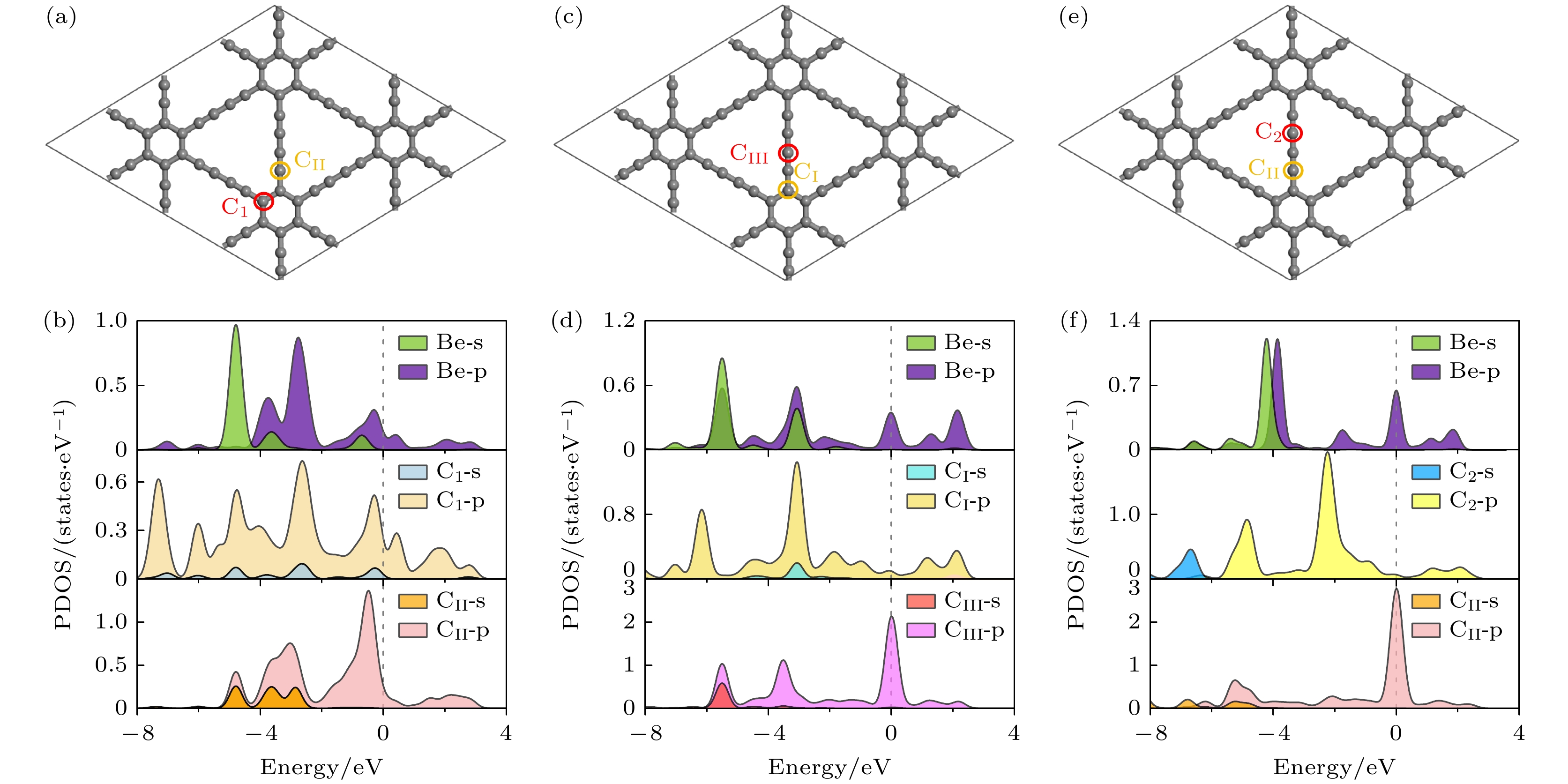
Fig. 8. (a) Top view and (b) side view of stable adsorption sites of a single Li atom at the CI site doped system; (c) top view and (d) side view at the CII site doped system; (e) top view and (f) side view at the CIII site doped system.
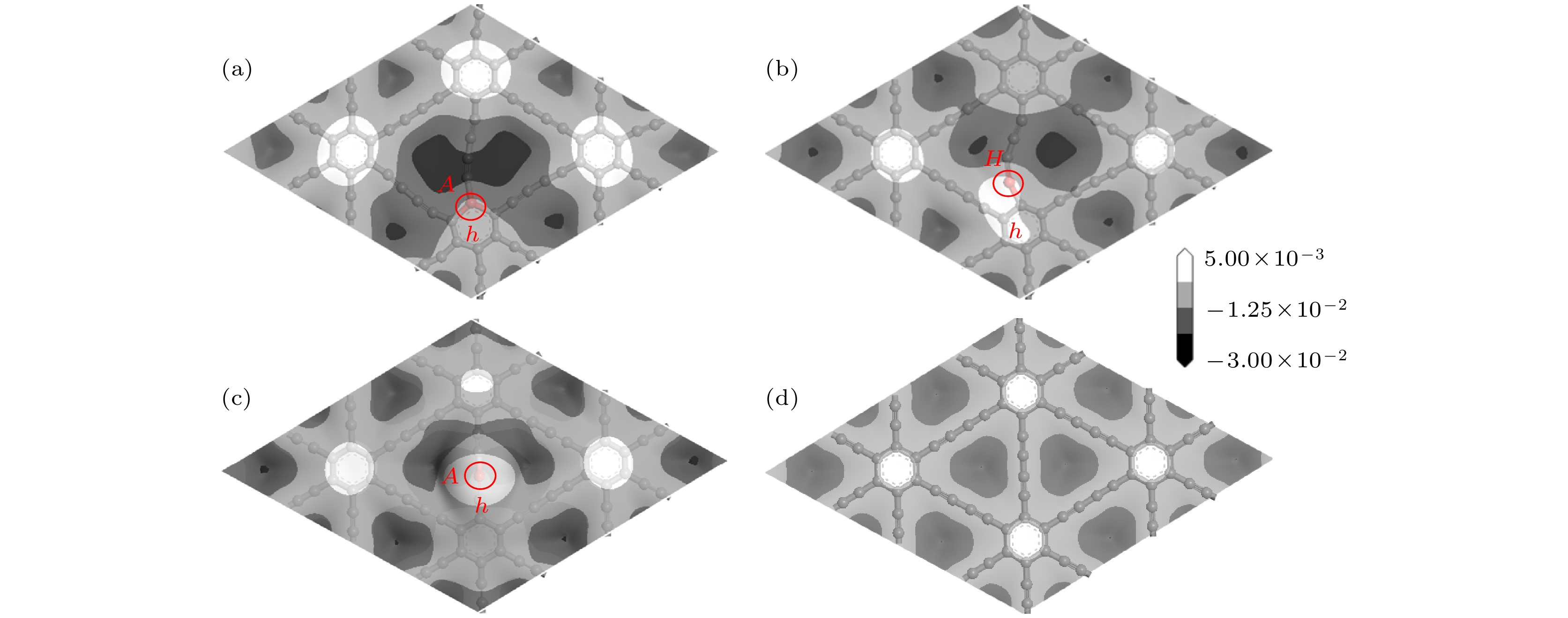
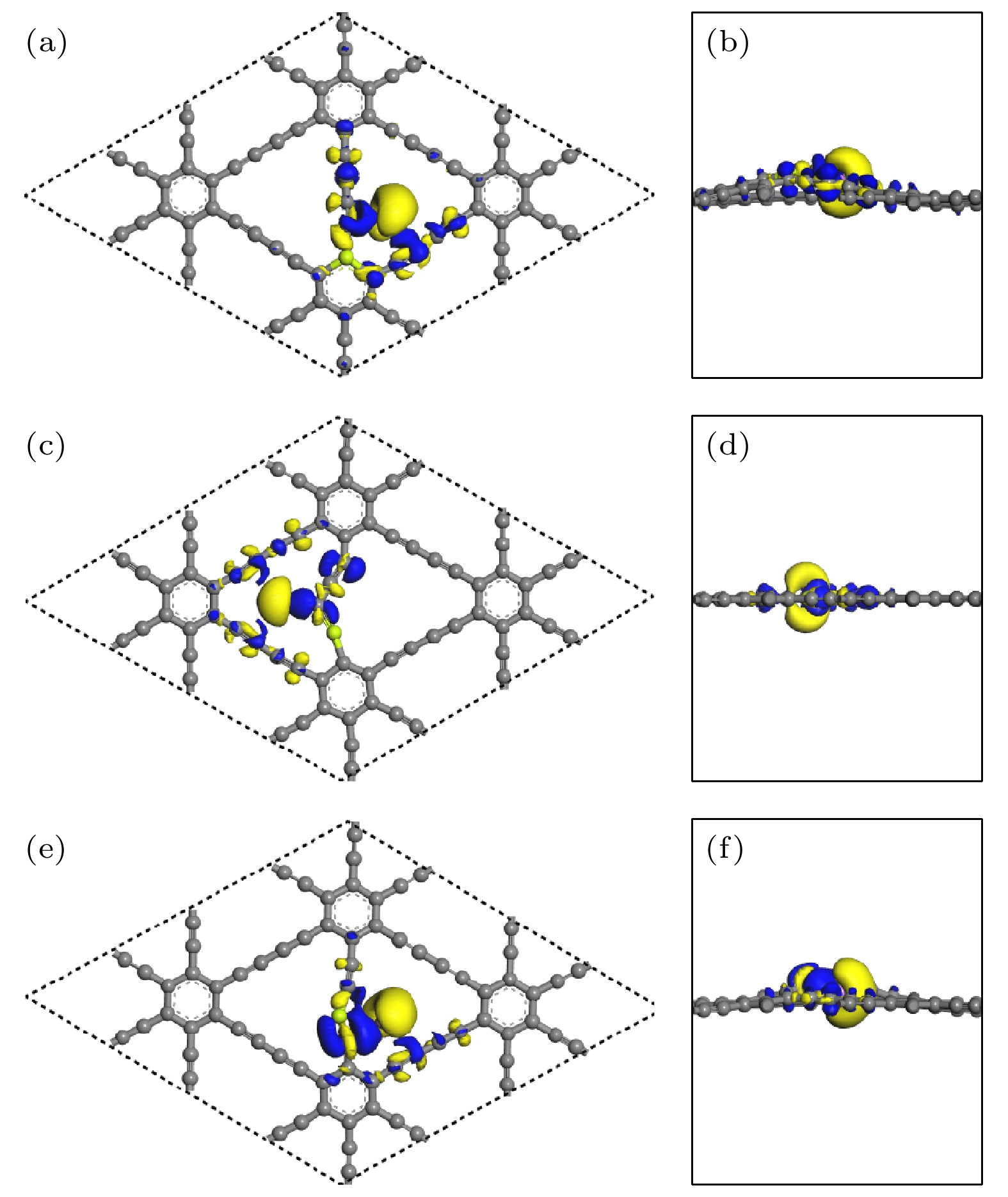
图 10 差分电荷密度图的(a), (c), (e)俯视图和(b), (d), (f)侧视图 (a), (b) Li吸附在CI位掺杂体系; (c), (d) Li吸附在CII位掺杂体系; (e), (f) Li吸附在CIII位掺杂体系(等值面为0.011 |e|/Å3)
Fig. 10. (a), (c), (e) Top view and (b), (d), (f) side view of the different charge density: (a), (b) the adsorption of Li atom on the CI doped system; (c), (d) the adsorption of Li atom on CII doped system; (e), (f) the adsorption of Li atom on CIII doped system (isosurface = 0.011 |e|/Å3).
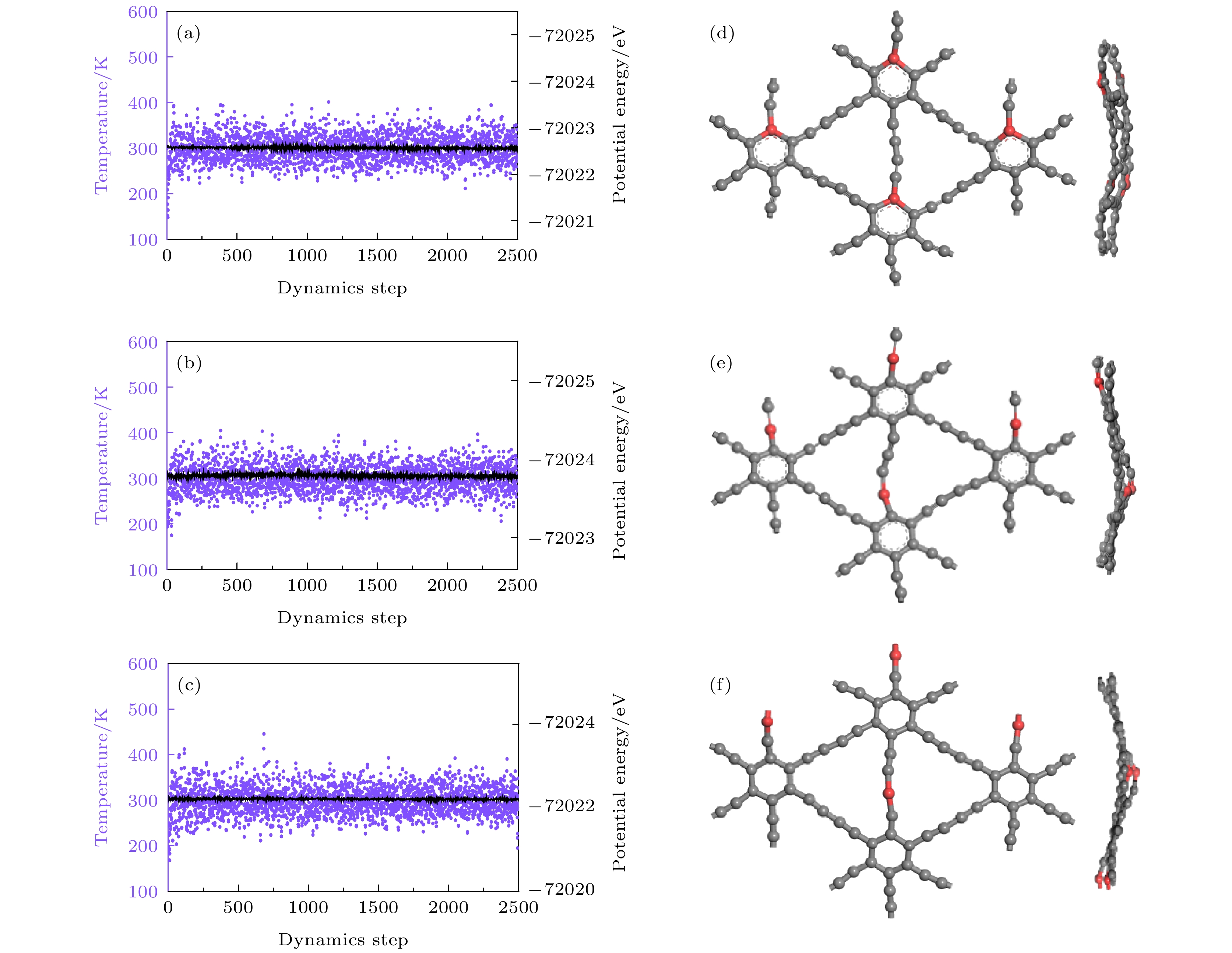
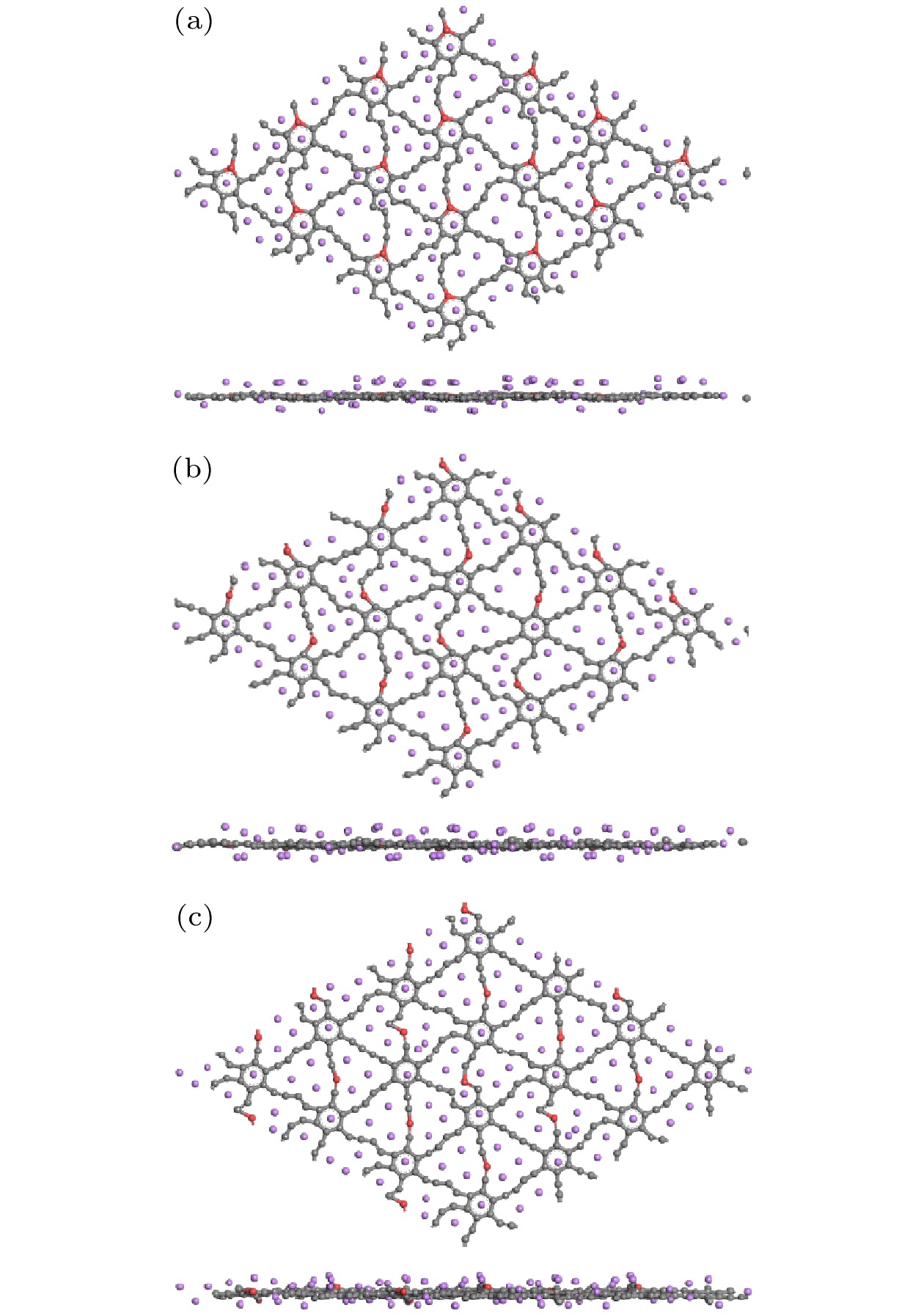
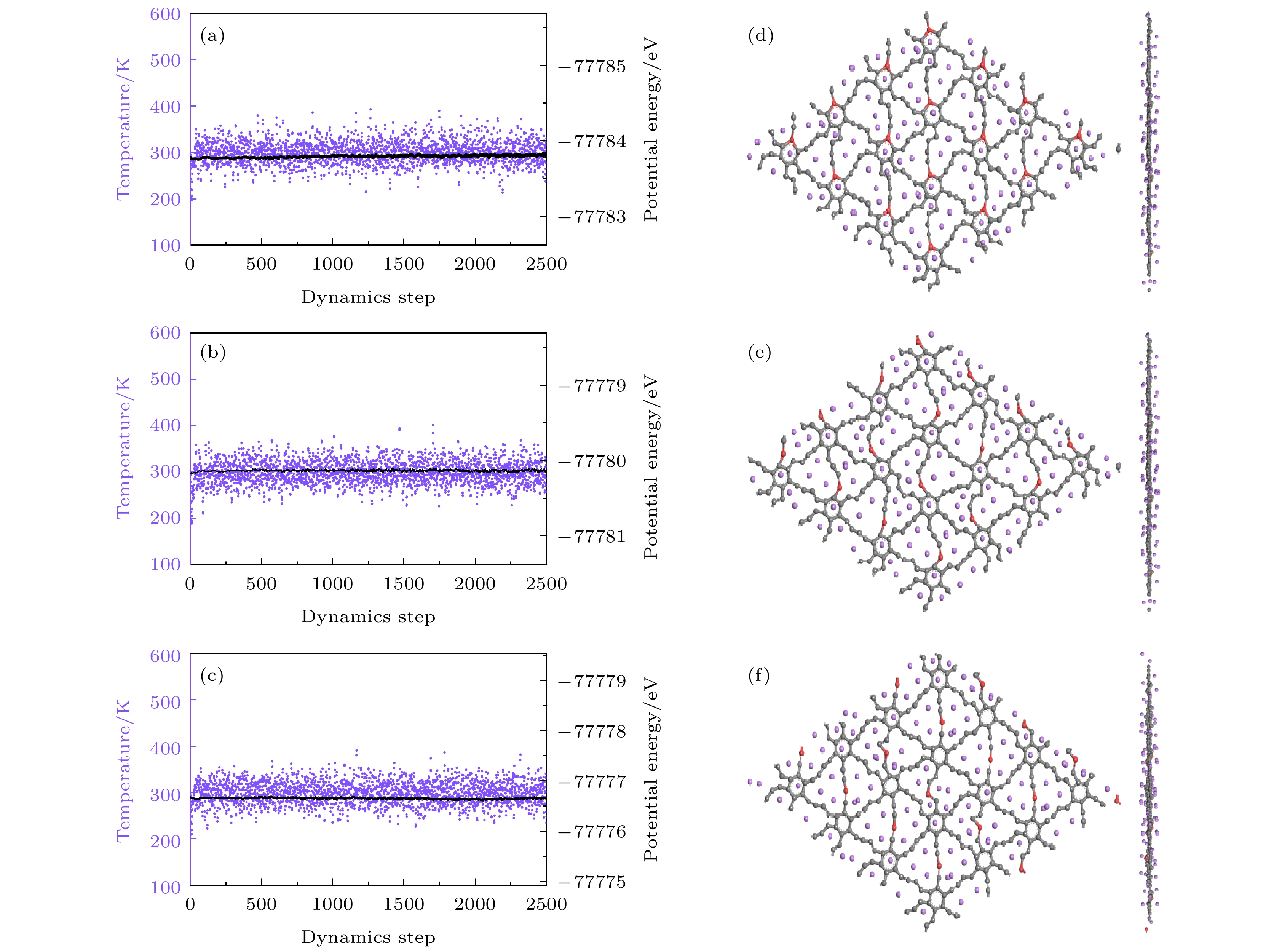
图 14 (a)—(c) Be掺杂石墨双炔最大Li容量的分子动力学结果以及(d)—(f)在300 K弛豫5 ps后的结构 (a), (d) CI位; (b), (e) CII位; (c), (f) CIII位
Fig. 14. (a)–(c) Molecular dynamics results of the maximum Li capacities of Be doped graphdiyne and (d)–(f) the structure after 5 ps of relaxation at 300 K: (a), (d) CI site; (b), (e) CII site; (c), (f) CIII site.
图 15 Li在Be掺杂石墨双炔上的扩散路径(第1和第3行)和对应的能量曲线图(第2和第4行) (a)—(d) CI位替换掺杂; (e)—(h) CII位替换掺杂; (i)—(l) CIII位替换掺杂
Fig. 15. Diffusion path (the first and the third rows) and corresponding energy curve (the second and the fourth rows) of Li on Be doped graphdiyne: (a)–(d) CI site doping; (e)–(h) CII site doping; (i)–(l) CIII site doping.
图 18 中锂高锂浓度的扩散路径和对应的能量曲线图 (a) 中锂扩散路径; (b) 中锂对应的势垒; (c) 高锂扩散路径; (d) 高锂对应的势垒
Fig. 18. Diffusion path and corresponding energy profiles for the medium-high lithium concentration: (a) Medium lithium diffusion path; (b) the barrier corresponding to the medium lithium; (c) high lithium diffusion path; (d) the barrier corresponding to the high lithium level.
表 1 CI, CII, CIII位掺杂不同浓度的Be原子构型形成能(单位: eV)
Table 1. Formation energies of Be atoms at different concentrations of CI, CII, CIII sites doping (unit: eV).
掺杂位 替换1个Be(1.39%) 替换2个Be(2.78%) 替换3个Be(4.17%) 替换4个Be(5.56%) CI 1.28 0.79 0.79 0.63 CII 0.83 0.36 0.40 0.25 CIII 1.04 0.80 0.81 0.66 表 2 不同掺杂浓度下内聚能(单位: eV)
Table 2. Cohesive energy at different doping concentrations (unit: eV).
掺杂位 替换1个Be(1.39%) 替换2个Be(2.78%) 替换3个Be(4.17%) 替换4个Be(5.56%) CI –7.816 –7.729 –7.636 –7.552 CII –7.822 –7.741 –7.652 –7.572 CIII –7.819 –7.729 –7.635 –7.550 -
[1] Erickson E M, Ghanty C, Aurbach D 2014 J. Phys. Chem. Lett. 5 3313
 Google Scholar
Google Scholar
[2] Novoselov K S, Geim A K, Morozov S V, Jiang D, Zhang Y, Dubonos S V, Grigorieva I V, Firsov A A 2004 Science 306 666
 Google Scholar
Google Scholar
[3] Yoo E, Kim J, Hosono E, Zhou H S, Kudo T, Honma I 2008 Nano Lett. 8 2277
 Google Scholar
Google Scholar
[4] Liu X, Wang C Z, Yao Y X, Lu W C, Hupalo M, Tringides M C, Ho K M 2011 Phys. Rev. B 83 235411
 Google Scholar
Google Scholar
[5] Li W, He Y H, Wang L, Ding G H, Zhang Z Q, Lortz R W, Sheng P, Wang N 2011 Phys. Rev. B 84 045431.
 Google Scholar
Google Scholar
[6] Liu X, Hupalo M, Wang C Z, Lu W C, Tringides M C 2012 Phys. Rev. B 86 081414
 Google Scholar
Google Scholar
[7] Baughman R H, Eckhardt H, Kertesz M 1987 J. Chem. Phys. 87 6687
 Google Scholar
Google Scholar
[8] Li G X, Li Y L, Liu H B, Guo Y B, Li Y J, Zhu D B 2010 Chem. Commun. 46 3256
 Google Scholar
Google Scholar
[9] Narita N, Nagai S, Suzuki S, Nakao K 1998 Phys. Rev. B 58 11009
 Google Scholar
Google Scholar
[10] Zhang H Y, Xia Y Y, Bu H X, Wang X P, Zhang M, Zhao L X, Luo T H, Zhao M W 2013 J. Appl. Phys. 113 044309
 Google Scholar
Google Scholar
[11] He J J, Wang N, Cui Z L, Du H P, Fu L, Huang C S, Yang Z, Shen X Y, Yi Y P, Tu Z Y, Li Y L 2017 Nat. Commun. 8 1172
 Google Scholar
Google Scholar
[12] Urbain F, Smirnov V, Becker J P, et al. 2016 Energ. Environ. Sci. 9 145
 Google Scholar
Google Scholar
[13] Wang N, He J J, Tu Z Y, Yang Z, Zhao F H, Li X D, Huang C D, Wang K, Jiu T G, Yi Y P, Li Y L 2017 Angew. Chem. Int. Edit. 56 10740
 Google Scholar
Google Scholar
[14] Shen X Y, Li X D, Zhao F H, Wang N, Xie C P, He J J, Si W Y, Yi Y Y, Yang Z, Li X F, Lu F S, Huang C S 2019 2D Materials 6 035020
 Google Scholar
Google Scholar
[15] 曾雯2020 硕士学位论文 (重庆: 重庆大学)
Zeng W 2020 M. S. Thesis (Chongqing: Chongqing University
[16] Wang N, Li X D, Tu Z Y, Zhao F H, He J J, Guan Z Y, Huang C D, Yi Y P, Li Y L 2018 Angew. Chem. Int. Edit. 57 3968
 Google Scholar
Google Scholar
[17] Yang Z, Liu R R, Wang N, He J J, Wang K, Li X D, Shen X Y, Wang X, Lv Q, Zhang M J, Luo J, Jiu T G, Hou Z F, Huang C S 2018 Carbon 137 442
 Google Scholar
Google Scholar
[18] Yang Z, Shen X Y, Wang N, He J J, Li X D, Wang X, Hou Z F, Wang K, Gao J, Jiu T G, Huang C S 2019 ACS Appl. Mater. Interfaces 11 2608
 Google Scholar
Google Scholar
[19] Hussain A, Ullah S, Farhan M A 2016 RSC Adv. 6 55990
 Google Scholar
Google Scholar
[20] Ullah S, Hussain A, Syed W, Saqlain M A, Ahmad I, Leenaertse O, Karimf A 2015 RSC Adv. 5 55762
 Google Scholar
Google Scholar
[21] Kost F, Linsmeier Ch, Oberkofler M, Reinelt M, Balden M, Herrmann A, Lindig S 2009 J. Nucl. Mater. 390–391 975
 Google Scholar
Google Scholar
[22] Goldstraß P, Linsmeier C 2000 Nucl. Instrum. Meth. B 161–163 411
 Google Scholar
Google Scholar
[23] Anghel A, Porosnicu C, Lungu C P, Sugiyama K, Krieger C, Roth J 2011 J. Nucl. Mater. 416 9
 Google Scholar
Google Scholar
[24] Ferro Y, Allouche A, Linsmeier C 2013 J. Appl. Phys. 113 213514
 Google Scholar
Google Scholar
[25] Campbell A, Cakmak E, Henry B, et al. 2023 Be 2C Synthesis, Properties, and Ion-beam Irradiation Damage Characterization ORNL/TM-2023/3011
[26] López-Urías F, Terrones M, Terrones H 2015 Carbon 84 317
 Google Scholar
Google Scholar
[27] Ullah S, Denis P A, Sato F 2017 Appl. Mater. Today 9 333
 Google Scholar
Google Scholar
[28] Becke A D 1988 Phys. Rev. A 38 3098
 Google Scholar
Google Scholar
[29] Delley B 1990 J. Chem. Phys. 92 508
 Google Scholar
Google Scholar
[30] Perdew J P, Burke K, Ernzerhof M 1996 Phys. Rev. Lett. 77 3865
 Google Scholar
Google Scholar
[31] Long M Q, Tang L, Wang D, Li Y L, Shuai Z G 2011 ACS Nano 5 2593
 Google Scholar
Google Scholar
[32] Sun C, Searles D J 2012 J. Phys. Chem. C 116 26222
 Google Scholar
Google Scholar
[33] Hwang H J, Koo J, Park M, Park N, Kwon Y, Lee H 2013 J. Phys. Chem. C 117 6919
 Google Scholar
Google Scholar
[34] Zheng F C, Yang Y, Chen Q W 2014 Nat. Commun. 5 5261
 Google Scholar
Google Scholar
[35] Eftekhari A 2017 Energy Storage Mater. 7 157
 Google Scholar
Google Scholar
[36] 蔡梦圆, 唐春梅, 张秋月 2019 68 213601
 Google Scholar
Google Scholar
Cai M Y, Tang C M, Zhang Q Y 2019 Acta Phys. Sin. 68 213601
 Google Scholar
Google Scholar
[37] Jang B, Koo J, Park M, Lee H, Nam J, Kwon Y, Lee H 2013 Appl. Phys. Lett. 103 263904
 Google Scholar
Google Scholar
计量
- 文章访问数: 2747
- PDF下载量: 85
- 被引次数: 0













 下载:
下载: