-
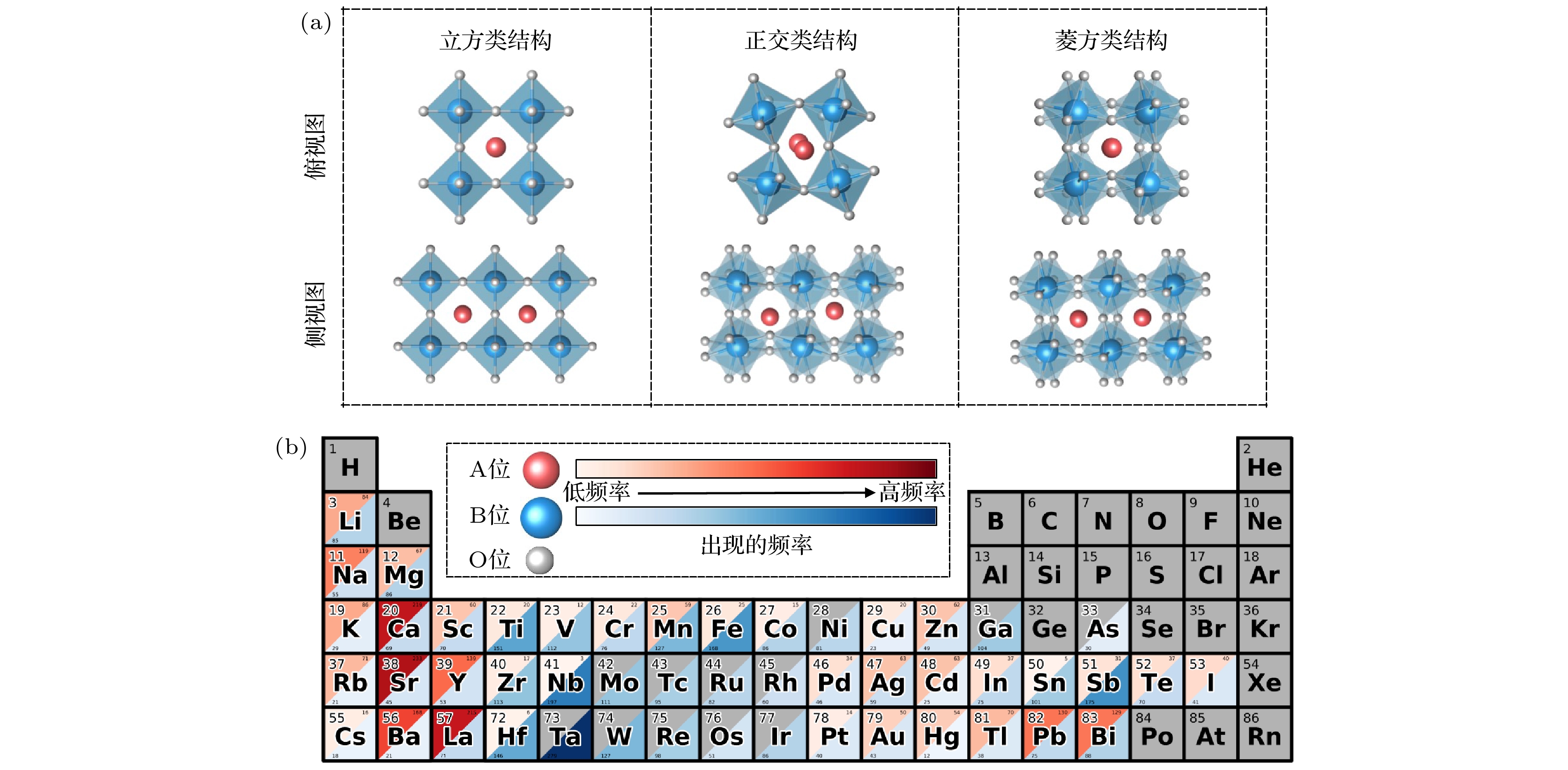
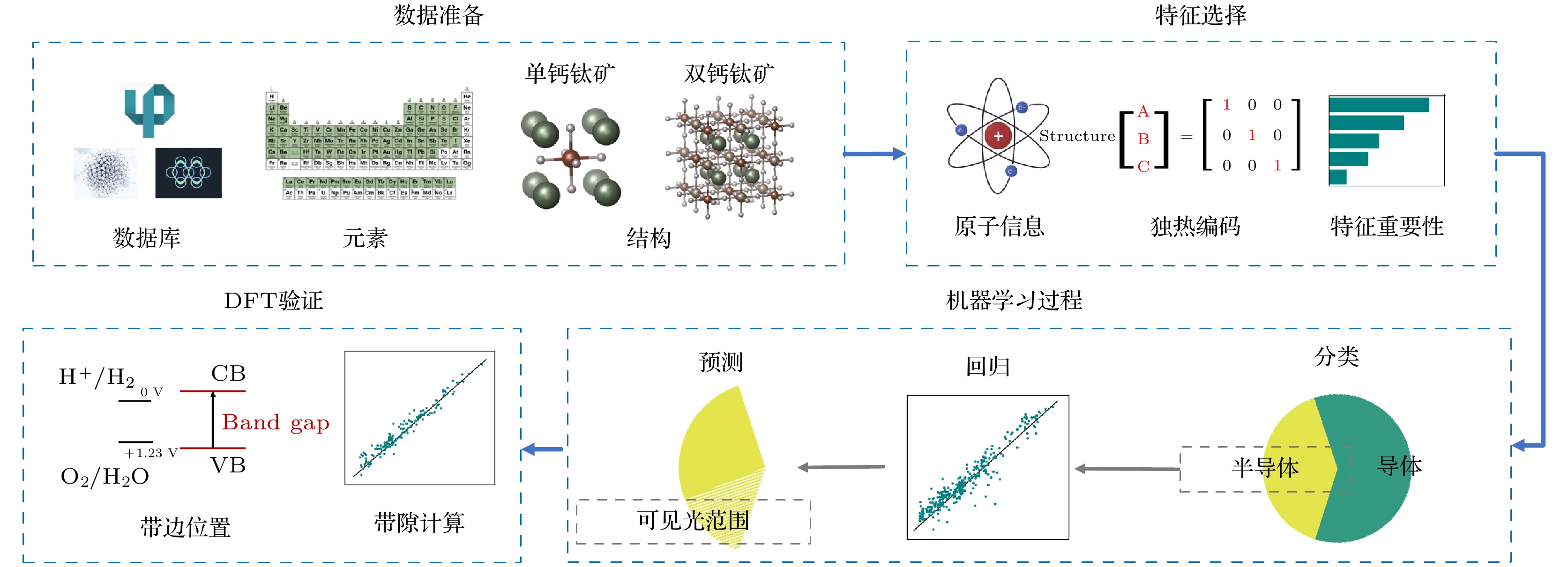
A2BB'O6型双钙钛矿氧化物材料, 相比于ABO3型单钙钛矿氧化物材料, 具有更好的稳定性和更宽泛的能带选择范围, 在光催化全解水领域具有良好的应用前景. 然而, 由于晶体结构和组成元素的多样性, 实验和理论上快速、准确搜寻高催化活性的A2BB'O6型双钙钛矿氧化物材料具有相当大的挑战性. 本文由材料数据库的带隙值数据出发, 采用机器学习与第一性原理相结合的方法, 从50000多种A2BB'O6型双钙钛矿氧化物材料中筛选出近8000种可能适用于光催化全解水的材料. 对筛选结果的统计分析表明, B/B'位均为d10金属离子的双钙钛矿氧化物, 更有可能成为全解水光催化剂. 随后通过进一步的第一性原理计算挑选出Sr2GaSbO6, Sr2InSbO6和K2NbTaO6这3种带边位置合适且不含铅、汞离子的A2BB'O6型双钙钛矿氧化物材料作为候选的全解水光催化剂.Double perovskite oxide A2BB'O6 has better stability and wider bandgap range than ABO3-type oxide, and exhibits great prospects in photocatalytic overall water splitting. However, owing to the diversity of crystal structure and constituents of perovskite oxide, rapidly and accurately searching for A2BB'O6 for photocatalyst is still a big challenge, both experimentally and theoretically. In this work, in order to screen out suitable double perovskite oxide photocatalysts, a multi-step framework combined with machine learning technique and first-principles calculations is proposed. Nearly 8000 candidates with proper bandgaps for water splitting are screened out from among more than 50000 A2BB'O6-type double perovskite oxides. Statistical analysis of the results shows that double perovskite oxides with d10 metal ions at B/B' sites are more likely to have good absorption of visible light, and the structural symmetry of double perovskite also has influence on the bandgap value. Furthermore, first-principles calculations demonstrate that Sr2GaSbO6, Sr2InSbO6 and K2NbTaO6 are non-toxic photocatalyst candidates with proper band edges for overall water splitting.
-
Keywords:
- machine learning /
- double perovskite oxides /
- photocatalysis /
- overall water splitting /
- first-principle method
[1] Dorian J P, Franssen H T, Simbeck D R 2006 Energy Policy 34 1984
 Google Scholar
Google Scholar
[2] Omer A M 2008 Renew. Sust. Energ. Rev. 12 2265
 Google Scholar
Google Scholar
[3] Pfenninger S, Hawkes A, Keirstead J 2014 Renew. Sust. Energ. Rev. 33 74
 Google Scholar
Google Scholar
[4] Liang K, Huang T, Yang K, Si Y, Wu H Y, Lian J C, Huang W Q, Hu W Y, Huang G F 2021 Phys. Rev. Appl. 16 054043
 Google Scholar
Google Scholar
[5] Ameen S, Rub M A, Kosa S A, Alamry K A, Akhtar M S, Shin H S, Seo H K, Asiri A M, Nazeeruddin M K 2016 ChemSusChem 9 10
 Google Scholar
Google Scholar
[6] Chen S, Takata T, Domen K 2017 Nat. Rev. Mater. 2 17050
 Google Scholar
Google Scholar
[7] Hisatomi T, Kubota J, Domen K 2014 Chem. Soc. Rev. 43 7520
 Google Scholar
Google Scholar
[8] Maeda K, Domen K 2010 J. Phys. Chem. Lett. 1 2655
 Google Scholar
Google Scholar
[9] Kumar A, Kumar A, Krishnan V 2020 ACS Catal. 10 10253
 Google Scholar
Google Scholar
[10] Peña M A, Fierro J L G 2001 Chem. Rev. 101 1981
 Google Scholar
Google Scholar
[11] Ouyang Y, Li Y, Zhu P, Li Q, Gao Y, Tong J, Shi L, Zhou Q, Ling C, Chen Q, Deng Z, Tan H, Deng W, Wang J 2019 J. Mater. Chem. A 7 2275
 Google Scholar
Google Scholar
[12] Grimaud A, May K J, Carlton C E, Lee Y L, Risch M, Hong W T, Zhou J, Shao-Horn Y 2013 Nat. Commun. 4 2439
 Google Scholar
Google Scholar
[13] Yin W, Weng B, Ge J, Sun Q, Li Z, Yan Y 2019 Energy Environ. Sci. 12 442
 Google Scholar
Google Scholar
[14] Sun H, Xu X, Song Y, Zhou W, Shao Z 2021 Adv. Funct. Mater. 31 2009779
 Google Scholar
Google Scholar
[15] Aczel A A, Bugaris D E, Li L, Yan J, de la Cruz C, zur Loye H C, Nagler S E 2013 Phys. Rev. B 87 014435
 Google Scholar
Google Scholar
[16] Zhou Q, Lu S, Wu Y, Wang J 2020 J. Phys. Chem. Lett. 11 3920
 Google Scholar
Google Scholar
[17] Lu S, Zhou Q, Guo Y, Wang J 2022 Chem 8 769
 Google Scholar
Google Scholar
[18] Lu S, Zhou Q, Guo Y, Zhang Y, Wu Y, Wang J 2020 Adv. Mater. 32 2002658
 Google Scholar
Google Scholar
[19] Wu Y, Lu S, Ju M, Zhou Q, Wang J 2021 Nanoscale 13 12250
 Google Scholar
Google Scholar
[20] Goldsmith B R, Esterhuizen J, Liu J, Bartel C J, Sutton C 2018 AlChE J. 64 2311
 Google Scholar
Google Scholar
[21] Chen T, Guestrin C 2016 XGBoost: A Scalable Tree Boosting System (Association for Computing Machinery) pp785–794
[22] Natekin A, Knoll A 2013 Front. Neurorob. 7
[23] Hafner J 2008 J. Comput. Chem. 29 2044
 Google Scholar
Google Scholar
[24] Perdew J P, Burke K, Ernzerhof M 1996 Phys. Rev. Lett. 77 3865
 Google Scholar
Google Scholar
[25] Cai B, Chen X, Xie M, Zhang S, Liu X, Yang J, Zhou W, Guo S, Zeng H 2018 Mater. Horiz. 5 961
 Google Scholar
Google Scholar
[26] Blöchl P E 1994 Phys. Rev. B 50 17953
 Google Scholar
Google Scholar
[27] Monkhorst H J, Pack J D 1976 Phys. Rev. B 13 5188
 Google Scholar
Google Scholar
[28] Aryasetiawan F, Karlsson K, Jepsen O, Schönberger U 2006 Phys. Rev. B 74 125106
 Google Scholar
Google Scholar
[29] Becke A D 1993 J. Chem. Phys. 98 1372
 Google Scholar
Google Scholar
[30] Curtarolo S, Setyawan W, Hart G L W, Jahnatek M, Chepulskii R V, Taylor R H, Wang S, Xue J, Yang K, Levy O, Mehl M J, Stokes H T, Demchenko D O, Morgan D 2012 Com. Mat. Sci. 58 218
 Google Scholar
Google Scholar
[31] Saal J E, Kirklin S, Aykol M, Meredig B, Wolverton C 2013 JOM 65 1501
 Google Scholar
Google Scholar
[32] Jain A, Ong S P, Hautier G, Chen W, Richards W D, Dacek S, Cholia S, Gunter D, Skinner D, Ceder G, Persson K A 2013 APL Mater. 1 011002
 Google Scholar
Google Scholar
[33] Goldschmidt V M 1926 Naturwissenschaften 14 477
 Google Scholar
Google Scholar
[34] Sun Q, Yin W 2017 J. Am. Chem. Soc. 139 14905
 Google Scholar
Google Scholar
[35] Bartel C J, Sutton C, Goldsmith B R, Ouyang R, Musgrave C B, Ghiringhelli L M, Scheffler M 2019 Sci. Adv. 5 eaav0693
 Google Scholar
Google Scholar
[36] Weng B, Song Z, Zhu R, Yan Q, Sun Q, Grice C G, Yan Y, Yin W 2020 Nat. Commun. 11 3513
 Google Scholar
Google Scholar
[37] Filip-Marina R, Giustino F 2018 Proc. Natl. Acad. Sci. U. S. A. 115 5397
 Google Scholar
Google Scholar
[38] Ye W, Chen C, Dwaraknath S, Jain A, Ong S P, Persson K A 2018 MRS Bull. 43 664
 Google Scholar
Google Scholar
[39] Zhao X G, Yang J H, Fu Y, Yang D, Xu Q, Yu L, Wei S H, Zhang L 2017 J. Am. Chem. Soc. 139 2630
 Google Scholar
Google Scholar
[40] Lu S, Zhou Q, Ma L, Guo Y, Wang J 2019 Small Methods 3 1900360
 Google Scholar
Google Scholar
[41] Goodenough J B 2004 Rep. Prog. Phys. 67 1915
 Google Scholar
Google Scholar
[42] Okada S, Ohzeki M, Taguchi S 2019 Sci. Rep. 9 13036
 Google Scholar
Google Scholar
[43] Wahl R, Vogtenhuber D, Kresse G 2008 Phys. Rev. B 78 104116
 Google Scholar
Google Scholar
[44] Liu P, Nisar J, Pathak B, Ahuja R 2012 Int. J. Hydrogen Energy 37 11611
 Google Scholar
Google Scholar
[45] Chou H, Hwang B, Sun C 2013 New and Future Developments in Catalysis (Amsterdam: Elsevier) pp217–270
[46] Inoue Y 2009 Energy Environ. Sci. 2 364
 Google Scholar
Google Scholar
[47] Kudo A, Hijii S 1999 Chem. Lett. 28 1103
 Google Scholar
Google Scholar
[48] Kudo A, Miseki Y 2009 Chem. Soc. Rev. 38 253
 Google Scholar
Google Scholar
[49] Acar C, Dincer I, Naterer G F 2016 Int. J. Energy Res. 40 1449
 Google Scholar
Google Scholar
[50] Kaspar T C, Sushko P V, Spurgeon S R, Bowden M E, Keavney D J, Comes R B, Saremi S, Martin L, Chambers S A 2019 Adv. Mater. Interfaces 6 1801428
 Google Scholar
Google Scholar
[51] Greiner M T, Helander M G, Tang W, Wang Z B, Qiu J, Lu Z 2012 Nat. Mater. 11 76
 Google Scholar
Google Scholar
[52] El-Sayed A, Borghetti P, Goiri E, Rogero C, Floreano L, Lovat G, Mowbray D J, Cabellos J L, Wakayama Y, Rubio A, Ortega J E, de Oteyza D G 2013 ACS Nano 7 6914
 Google Scholar
Google Scholar
-
图 3 (a) 分类模型中重要性前十的特征和混淆矩阵; (b)分类模型测试集ROC曲线和AUC值; (c)回归模型中重要性前十的特征; (d)回归模型测试集R2, 均方误差, 平均绝对误差和解释方差
Fig. 3. (a) Relative feature importance of top 10 most important features and confusion matrix for bandgap classification; (b) receiver operating characteristic (ROC) curve for bandgap classification test set, area under the ROC curve (AUC) is provided; (c) relative feature importance of top 10 most important features for bandgap regression; (d) performance of bandgap regression model, coefficient of determination (R2), mean square error (MSE), mean absolute error (MAE) and explained variance (EV) are provided.
图 4 (a) 预测的钙钛矿带隙值百分比统计图, 红色区域的代表双钙钛矿的 B/B' 位都是d0或d10金属离子, 灰色区域代表B/B' 位点中只有一个是 d0 或 d10 金属离子, 蓝色区域代表B/B' 位点不含d0 或 d10 金属离子; (b) B/B' 位点都是d0 或d10金属离子的双钙钛矿带隙分布图, 绿色区域为可见光能量范围; (c)不同B/B' 位组分下双钙钛矿带隙统计图; (d)相同化学式下, 3种晶系结构的双钙钛矿带隙值, 其中B/B' 位都是d0或d10金属离子
Fig. 4. (a) The percentage chart of predict set of bandgap values with the percentage of perovskites, red represents all B/B' sites are d0 or d10 metal ions, grey represents only one of B/B' sites is d0 or d10 metal ion, blue represents none of B/B' sites are d0 or d10 metal ion; (b) the perovskite bandgap distribution diagram, colored area represents visible light energy range; (c) pie chart of the distribution ratio of different B/B' site ions; (d) comparison of bandgap values of 3 different structures, B/B' sites are all with d0 or d10 metal ions.
图 5 (a) 29种菱方类结构双钙钛矿材料DFT带隙与机器学习预测带隙的比较; (b) 3种候选双钙钛矿相对于水的氧化还原势的HSE带边位置, 以及作为比较基准的SrTiO3 (立方相)带边位置
Fig. 5. (a) DFT bandgap verification of 29 rhombohedral double perovskites in the prediction set; (b) the HSE band edge positions with respect to the water reduction and oxidation potential levels of selected double perovskites. SrTiO3 (cubic) is listed as a benchmark.
表 A1 过渡金属计算时附加的Hubbard U值
Table A1. Hubbard U value for the transition metal elements.
元素 U 值 V 3.25 Mo 4.38 W 6.2 Ni 6.2 Mn 3.9 Fe 5.3 Cr 3.7 Co 3.32 表 A2 特征符号及含义
Table A2. The symbol of features and their corresponding meanings.
符号 含义 Na 原子数 Ra 原子半径 Va 原子体积 Rcov 共价半径 Ea 电子亲合能 Ne 电子数 χ 鲍林电负性 Hf 形成热 Nm 门捷列夫数 Np 周期 Rion 离子半径 t 容忍因子 表 1 3种候选双钙钛矿材料的PBE带隙、HSE带隙及带隙类型
Table 1. PBE and HSE bandgap of three kinds of double perovskite candidates and their bandgap categories.
Formula Eg-PBE /eV Eg-HSE /eV Bandgap Sr2GaSbO6 1.37 2.80 indirect Sr2InSbO6 1.63 3.07 indirect K2NbTaO6 1.77 3.06 direct -
[1] Dorian J P, Franssen H T, Simbeck D R 2006 Energy Policy 34 1984
 Google Scholar
Google Scholar
[2] Omer A M 2008 Renew. Sust. Energ. Rev. 12 2265
 Google Scholar
Google Scholar
[3] Pfenninger S, Hawkes A, Keirstead J 2014 Renew. Sust. Energ. Rev. 33 74
 Google Scholar
Google Scholar
[4] Liang K, Huang T, Yang K, Si Y, Wu H Y, Lian J C, Huang W Q, Hu W Y, Huang G F 2021 Phys. Rev. Appl. 16 054043
 Google Scholar
Google Scholar
[5] Ameen S, Rub M A, Kosa S A, Alamry K A, Akhtar M S, Shin H S, Seo H K, Asiri A M, Nazeeruddin M K 2016 ChemSusChem 9 10
 Google Scholar
Google Scholar
[6] Chen S, Takata T, Domen K 2017 Nat. Rev. Mater. 2 17050
 Google Scholar
Google Scholar
[7] Hisatomi T, Kubota J, Domen K 2014 Chem. Soc. Rev. 43 7520
 Google Scholar
Google Scholar
[8] Maeda K, Domen K 2010 J. Phys. Chem. Lett. 1 2655
 Google Scholar
Google Scholar
[9] Kumar A, Kumar A, Krishnan V 2020 ACS Catal. 10 10253
 Google Scholar
Google Scholar
[10] Peña M A, Fierro J L G 2001 Chem. Rev. 101 1981
 Google Scholar
Google Scholar
[11] Ouyang Y, Li Y, Zhu P, Li Q, Gao Y, Tong J, Shi L, Zhou Q, Ling C, Chen Q, Deng Z, Tan H, Deng W, Wang J 2019 J. Mater. Chem. A 7 2275
 Google Scholar
Google Scholar
[12] Grimaud A, May K J, Carlton C E, Lee Y L, Risch M, Hong W T, Zhou J, Shao-Horn Y 2013 Nat. Commun. 4 2439
 Google Scholar
Google Scholar
[13] Yin W, Weng B, Ge J, Sun Q, Li Z, Yan Y 2019 Energy Environ. Sci. 12 442
 Google Scholar
Google Scholar
[14] Sun H, Xu X, Song Y, Zhou W, Shao Z 2021 Adv. Funct. Mater. 31 2009779
 Google Scholar
Google Scholar
[15] Aczel A A, Bugaris D E, Li L, Yan J, de la Cruz C, zur Loye H C, Nagler S E 2013 Phys. Rev. B 87 014435
 Google Scholar
Google Scholar
[16] Zhou Q, Lu S, Wu Y, Wang J 2020 J. Phys. Chem. Lett. 11 3920
 Google Scholar
Google Scholar
[17] Lu S, Zhou Q, Guo Y, Wang J 2022 Chem 8 769
 Google Scholar
Google Scholar
[18] Lu S, Zhou Q, Guo Y, Zhang Y, Wu Y, Wang J 2020 Adv. Mater. 32 2002658
 Google Scholar
Google Scholar
[19] Wu Y, Lu S, Ju M, Zhou Q, Wang J 2021 Nanoscale 13 12250
 Google Scholar
Google Scholar
[20] Goldsmith B R, Esterhuizen J, Liu J, Bartel C J, Sutton C 2018 AlChE J. 64 2311
 Google Scholar
Google Scholar
[21] Chen T, Guestrin C 2016 XGBoost: A Scalable Tree Boosting System (Association for Computing Machinery) pp785–794
[22] Natekin A, Knoll A 2013 Front. Neurorob. 7
[23] Hafner J 2008 J. Comput. Chem. 29 2044
 Google Scholar
Google Scholar
[24] Perdew J P, Burke K, Ernzerhof M 1996 Phys. Rev. Lett. 77 3865
 Google Scholar
Google Scholar
[25] Cai B, Chen X, Xie M, Zhang S, Liu X, Yang J, Zhou W, Guo S, Zeng H 2018 Mater. Horiz. 5 961
 Google Scholar
Google Scholar
[26] Blöchl P E 1994 Phys. Rev. B 50 17953
 Google Scholar
Google Scholar
[27] Monkhorst H J, Pack J D 1976 Phys. Rev. B 13 5188
 Google Scholar
Google Scholar
[28] Aryasetiawan F, Karlsson K, Jepsen O, Schönberger U 2006 Phys. Rev. B 74 125106
 Google Scholar
Google Scholar
[29] Becke A D 1993 J. Chem. Phys. 98 1372
 Google Scholar
Google Scholar
[30] Curtarolo S, Setyawan W, Hart G L W, Jahnatek M, Chepulskii R V, Taylor R H, Wang S, Xue J, Yang K, Levy O, Mehl M J, Stokes H T, Demchenko D O, Morgan D 2012 Com. Mat. Sci. 58 218
 Google Scholar
Google Scholar
[31] Saal J E, Kirklin S, Aykol M, Meredig B, Wolverton C 2013 JOM 65 1501
 Google Scholar
Google Scholar
[32] Jain A, Ong S P, Hautier G, Chen W, Richards W D, Dacek S, Cholia S, Gunter D, Skinner D, Ceder G, Persson K A 2013 APL Mater. 1 011002
 Google Scholar
Google Scholar
[33] Goldschmidt V M 1926 Naturwissenschaften 14 477
 Google Scholar
Google Scholar
[34] Sun Q, Yin W 2017 J. Am. Chem. Soc. 139 14905
 Google Scholar
Google Scholar
[35] Bartel C J, Sutton C, Goldsmith B R, Ouyang R, Musgrave C B, Ghiringhelli L M, Scheffler M 2019 Sci. Adv. 5 eaav0693
 Google Scholar
Google Scholar
[36] Weng B, Song Z, Zhu R, Yan Q, Sun Q, Grice C G, Yan Y, Yin W 2020 Nat. Commun. 11 3513
 Google Scholar
Google Scholar
[37] Filip-Marina R, Giustino F 2018 Proc. Natl. Acad. Sci. U. S. A. 115 5397
 Google Scholar
Google Scholar
[38] Ye W, Chen C, Dwaraknath S, Jain A, Ong S P, Persson K A 2018 MRS Bull. 43 664
 Google Scholar
Google Scholar
[39] Zhao X G, Yang J H, Fu Y, Yang D, Xu Q, Yu L, Wei S H, Zhang L 2017 J. Am. Chem. Soc. 139 2630
 Google Scholar
Google Scholar
[40] Lu S, Zhou Q, Ma L, Guo Y, Wang J 2019 Small Methods 3 1900360
 Google Scholar
Google Scholar
[41] Goodenough J B 2004 Rep. Prog. Phys. 67 1915
 Google Scholar
Google Scholar
[42] Okada S, Ohzeki M, Taguchi S 2019 Sci. Rep. 9 13036
 Google Scholar
Google Scholar
[43] Wahl R, Vogtenhuber D, Kresse G 2008 Phys. Rev. B 78 104116
 Google Scholar
Google Scholar
[44] Liu P, Nisar J, Pathak B, Ahuja R 2012 Int. J. Hydrogen Energy 37 11611
 Google Scholar
Google Scholar
[45] Chou H, Hwang B, Sun C 2013 New and Future Developments in Catalysis (Amsterdam: Elsevier) pp217–270
[46] Inoue Y 2009 Energy Environ. Sci. 2 364
 Google Scholar
Google Scholar
[47] Kudo A, Hijii S 1999 Chem. Lett. 28 1103
 Google Scholar
Google Scholar
[48] Kudo A, Miseki Y 2009 Chem. Soc. Rev. 38 253
 Google Scholar
Google Scholar
[49] Acar C, Dincer I, Naterer G F 2016 Int. J. Energy Res. 40 1449
 Google Scholar
Google Scholar
[50] Kaspar T C, Sushko P V, Spurgeon S R, Bowden M E, Keavney D J, Comes R B, Saremi S, Martin L, Chambers S A 2019 Adv. Mater. Interfaces 6 1801428
 Google Scholar
Google Scholar
[51] Greiner M T, Helander M G, Tang W, Wang Z B, Qiu J, Lu Z 2012 Nat. Mater. 11 76
 Google Scholar
Google Scholar
[52] El-Sayed A, Borghetti P, Goiri E, Rogero C, Floreano L, Lovat G, Mowbray D J, Cabellos J L, Wakayama Y, Rubio A, Ortega J E, de Oteyza D G 2013 ACS Nano 7 6914
 Google Scholar
Google Scholar
计量
- 文章访问数: 12124
- PDF下载量: 433
- 被引次数: 0













 下载:
下载: