-
热激活延迟荧光(TADF)作为一种特殊的分子荧光机制, 对于提高发光效率有着重要意义. 以C60和C70为代表的碳富勒烯具有高对称结构和离域π电子, 被广泛证明具有显著的TADF效应; 相比之下, 其他类富勒烯团簇的光物理性质尚不清楚. 本文利用含时密度泛函理论探索了一系列类富勒烯团簇的激发态性质, 包括实验合成的具有不同尺寸的氮化硼笼型团簇B12N12, B24N24和B36N36, 以及与B12N12结构相同、元素组成不同的B12P12, Al12N12和Ga12N12. 计算结果表明, 这些类富勒烯化合物团簇具有2.83—6.54 eV的能隙, 主要吸收紫外光, 荧光发射波长在可见光区间, 包括红光、橙光、蓝光和紫光. 它们的第一激发单重态和三重态的能量差较小(ΔEST < 0.29 eV), 因此可能通过系间窜越和反系间窜越发生TADF过程. 导致ΔEST较低的原因是这些化合物团簇的最高占据分子轨道和最低未占据分子轨道分布在不同的元素上, 使得二者重叠度较低. 这些理论结果揭示了类富勒烯团簇的发光性质和可能的荧光机理, 为寻找和设计稳定高效的发光材料提供了重要指导.Thermally activated delayed fluorescence (TADF), a unique molecular fluorescence mechanism, plays a key role in designing emitters of high efficiency. Carbon fullerenes such as C60 and C70 exhibit strong TADF with intensity even higher than that of the prompt fluorescence, owing to their long lifetimes of triplet state and modest singlet-triplet energy gaps. Thus, there arises the intriguing question whether other fullerene-like clusters can also have fluorescence and host the TADF effect. In this work, by time-dependent density functional theory (TD-DFT) calculations, we explore the excited-states of the experimentally reported boron nitride cage clusters B12N12, B24N24 and B36N36, as well as compound clusters B12P12, Al12N12 and Ga12N12 with the same geometry as B12N12. Using the HSE06 hybrid functional, the predicted energy gaps of these fullerene-like clusters are obtained to range from 2.83 eV to 6.54 eV. They mainly absorb ultraviolet light, and their fluorescence spectra are all in the visible range from 405.36 nm to 706.93 nm, including red, orange, blue, and violet emission colors. For the boron nitride cages, the energy gap of excited states increases with the cluster size increasing, accompanied by a blue shift of emission wavelength. For the clusters with B12N12 geometry and different elemental compositions, the excited energy gap decreases as the atomic radius increases, resulting in a red shift of emission wavelength. In addition, the highest occupied molecular orbitals (HOMOs) and lowest unoccupied molecular orbitals (LUMOs) of these compound cage clusters are distributed separately on different elements, resulting in small overlap between HOMO and LUMO wavefunctions. Consequently, these fullerene-like clusters exhibit small singlet-triplet energy differences below 0.29 eV, which is beneficial for the intersystem crossing between the excited singlet state and triplet state, and hence promoting the TADF process. Our theoretical results unveil the fluorescence characteristics of cage clusters other than carbon fullerenes, and provide important guidance for precisely modulating their emission colors by controlling the cluster sizes and elemental compositions. These experimentally feasible fullerene-like compound clusters possess many merits as fluorophors such as outstanding stabilities, non-toxicity, large energy gap, visible-light fluorescence, and small singlet-triplet energy gap. Therefore, they are promising luminescent materials for applications in display, sensors, biological detection and labelling, therapy, and medicine.
-
Keywords:
- fullerene-like clusters /
- excited singlet state /
- excited triplet state /
- fluorescence /
- reverse intersystem crossing /
- thermally activated delayed fluorescence
[1] Baleizao C, Berberan-Santos M N 2008 Ann. N. Y. Acad. Sci. 1130 224
 Google Scholar
Google Scholar
[2] Dos Santos P L, Etherington M K, Monkman A P 2018 J. Mater. Chem. C 6 4842
 Google Scholar
Google Scholar
[3] Parker C A, Hatchard C G 1961 Trans. Faraday Soc. 57 1894
 Google Scholar
Google Scholar
[4] Lam S K, Lo D 1997 Chem. Phys. Lett. 281 35
 Google Scholar
Google Scholar
[5] Wolf M W, Legg K D, Brown R E, Singer L A, Parks J H 1975 J. Am. Chem. Soc. 97 4490
 Google Scholar
Google Scholar
[6] Maciejewski A, Szymanski M 1986 J. Phys. Chem. 90 6314
 Google Scholar
Google Scholar
[7] Yusa S, Kamachi M, Morishima Y 1998 Photochem. Photobiol. 67 519
 Google Scholar
Google Scholar
[8] Nickel B, Klemp D 1993 Chem. Phys. 174 297
 Google Scholar
Google Scholar
[9] Nickel B, Klemp D 1993 Chem. Phys. 174 319
 Google Scholar
Google Scholar
[10] Uoyama H, Goushi K, Shizu K, Nomura H, Adachi C 2012 Nature 492 234
 Google Scholar
Google Scholar
[11] Sato K, Shizu K, Yoshimura K, Kawada A, Miyazaki H, Adachi C 2013 Phys. Rev. Lett. 110 247410
[12] Tanaka H, Shizu K, Miyazaki H, Adachi C 2012 Chem. Commun. 48 11392
 Google Scholar
Google Scholar
[13] Lee J, Shizu K, Tanaka H, Nomura H, Yasuda T, Adachi C 2013 J. Mater. Chem. C 1 4599
 Google Scholar
Google Scholar
[14] Liu L, Wei Q, Cheng Y, Ma H, Xiong S, Zhang X 2020 J. Mater. Chem. C 8 5839
 Google Scholar
Google Scholar
[15] Bachilo S M, Benedetto A F, Weisman R B, Nossal J R, Billups W E 2000 J. Phys. Chem. A 104 11265
 Google Scholar
Google Scholar
[16] Arbohast J W, Foote C S 1991 J. Am. Chem. Soc. 113 8886
 Google Scholar
Google Scholar
[17] Wasielewski M R, O'Neil M P, Lykke K R, Pellin M J, Gruen D M 1991 J. Am. Chem. Soc. 113 2774
 Google Scholar
Google Scholar
[18] Argentine S M, Kota K T, Francis A H 1995 J. Am. Chem. Soc. 117 11762
 Google Scholar
Google Scholar
[19] Berberan-Santos M N, Garcia J M M 1996 J. Am. Chem. Soc. 118 9391
 Google Scholar
Google Scholar
[20] Wang Y 1992 J. Phys. Chem. 96 764
 Google Scholar
Google Scholar
[21] Salazar F A, Fedorov A, Berberan-Santos M N 1997 Chem. Phys. Lett. 271 361
 Google Scholar
Google Scholar
[22] Anthony S M, Bachilo S M, Weisman R B 2003 J. Phys. Chem. A 107 10674
 Google Scholar
Google Scholar
[23] Li X 2007 Acta Phys. -Chim. Sin. 23 1792
 Google Scholar
Google Scholar
[24] Augusto V, Baleizão C, Berberan-Santos M N, Farinha J P S 2010 J. Mater. Chem. 20 1192
 Google Scholar
Google Scholar
[25] Zhao J, Du Q, Zhou S, Kumar V 2020 Chem. Rev. 120 9021
 Google Scholar
Google Scholar
[26] Zhao J, Ma L, Tian D, Xie R 2008 J. Comput. Theor. Nanosci. 5 7
[27] Oku T, Nishiwaki A, Narita I 2004 Sci. Technol. Adv. Mater. 5 635
[28] Oku T, Nishiwaki A, Narita I, Gonda M 2003 Chem. Phys. Lett. 380 620
 Google Scholar
Google Scholar
[29] Oku T, Nishiwaki A, Narita I 2004 Solid State Commun. 130 171
 Google Scholar
Google Scholar
[30] Oku T, Narita I, Nishiwaki A 2004 J. Phys. Chem. Solids 65 369
 Google Scholar
Google Scholar
[31] Oku T, Kuno Masaki, Narita I 2002 Diamond Relat. Mater. 11 940
 Google Scholar
Google Scholar
[32] Fowler P W, Heine T, Mitchell D, Schmidt R, Seifert G 1996 J. Chem. Soc. , Faraday Trans. 92 2197
 Google Scholar
Google Scholar
[33] Oku T, Hirano T, Kuno M, Kusunose T, Niihara K, Suganuma K 2000 Mater. Sci. Eng. B 74 206
[34] Runge E, Gross E K U 1984 Phys. Rev. Lett. 52 997
 Google Scholar
Google Scholar
[35] Marques M A L, Gross E K U 2004 Annu. Rev. Phys. Chem. 55 427
 Google Scholar
Google Scholar
[36] Frisch M, Trucks G, Schlegel H, et al. 2016 Gaussian 16 (Revision A. 03) (Wallingford, CT: Gaussian. Inc.)
[37] Hongzhiwei Technology, Device Studio, Version 2021 A, China. 2021. Available online: https://iresearch.net.cn/cloudSoftware (accessed on 21 January 2022)
[38] Shuai Z 2020 Chin. J. Chem. 38 1223
 Google Scholar
Google Scholar
[39] Wu X, Liang X, Du Q, et al. 2018 J. Phys. Condens. Matter 30 354002
 Google Scholar
Google Scholar
[40] Wu X, Zhou S, Huang X, Chen M, Bruce King R, Zhao J 2018 J. Comput. Chem. 39 2268
 Google Scholar
Google Scholar
[41] Sai L, Wu X, Gao N, Zhao J, Bruce King R 2017 Nanoscale 9 13905
 Google Scholar
Google Scholar
[42] Wu X, Sai L, Zhou S, Zhou P, Chen M, Springborg M, Zhao J 2020 Phys. Chem. Chem. Phys. 22 12959
 Google Scholar
Google Scholar
[43] Watanabe K, Taniguchi T, Kanda H 2004 Nat. Mater. 3 404
 Google Scholar
Google Scholar
[44] Lambrecht W R L, Segall B 1993 Phys. Rev. B 47 9289
 Google Scholar
Google Scholar
[45] Zhu Q, Guo X, Zhang J 2019 J. Comput. Chem. 40 1578
 Google Scholar
Google Scholar
[46] Peng Q, Fan D, Duan R, Yi Y, Niu Y, Wang D, Shuai Z 2017 J. Phys. Chem. C 121 13448
 Google Scholar
Google Scholar
[47] Vincenzo S, Pagliai M, Ciabini L, Cardini G 2001 J. Phys. Chem. A 105 11192
 Google Scholar
Google Scholar
[48] Kumar K S, Patnaik A 2010 J. Comput. Chem. 31 1182
[49] Chen X K, Tsuchiya Y, Ishikawa Y, Zhong C, Adachi C, Brédas J L 2017 Adv. Mater. 29 1702767
 Google Scholar
Google Scholar
[50] Taffet E J, Olivier Y, Lam F, Beljonne D, Scholes G D 2018 J. Phys. Chem. Lett. 9 1620
 Google Scholar
Google Scholar
[51] Saigo M, Miyata K, Tanaka S, Nakanotani H, Adachi C, Onda K 2019 J. Phys. Chem. Lett. 10 2475
 Google Scholar
Google Scholar
[52] Kasha M 1950 Discuss. Faraday Soc. 9 14
 Google Scholar
Google Scholar
[53] Yuan F, Yuan T, Sui L, Wang Z, Xi Z, Li Y, Li X, Fan L, Tan Z, Chen A, Jin M, Yang S 2018 Nat. Commun. 9 1
 Google Scholar
Google Scholar
[54] Palit D K, Sapre A V, Mittal J P, Rao C N R 1992 Chem. Phys. Lett. 195 1
 Google Scholar
Google Scholar
[55] Sun Y P, Wang P, Hamilton N B 1993 J. Am. Chem. Soc. 115 6378
 Google Scholar
Google Scholar
[56] Khandelwal P, Poddar P 2017 J. Mater. Chem. B 5 9055
 Google Scholar
Google Scholar
[57] Marcus R A 1956 J. Chem. Phys. 24 966
 Google Scholar
Google Scholar
[58] Marcus R A Sutin N 1985 Biochim. Biophys. Acta 811 265
 Google Scholar
Google Scholar
[59] Marcus R A 1993 Rev. Mod. Phys. 65 599
 Google Scholar
Google Scholar
[60] Samanta P K, Kim D, Coropceanu V, Brédas J L 2017 J. Am. Chem. Soc. 139 4042
[61] Gao X, Bai S, Fazzi D, Niehaus T, Barbatti M, Thiel W 2017 J. Chem. Theory Comput. 13 515
 Google Scholar
Google Scholar
[62] Liu Y, Lin M, Zhao Y 2017 J. Phys. Chem. A 121 1145
[63] Turro, N J 1991 Modern Molecular Photochemistry (Sausalito, CA: University Science Books) p29
[64] Lu T, Chen F 2012 J. Comput. Chem. 33 580
 Google Scholar
Google Scholar
[65] Liu Z, Lu T, Chen Q 2020 Carbon 165 461
 Google Scholar
Google Scholar
[66] Grabowski Z R, Rotkiewicz K 2003 Chem. Rev. 103 3899
 Google Scholar
Google Scholar
-
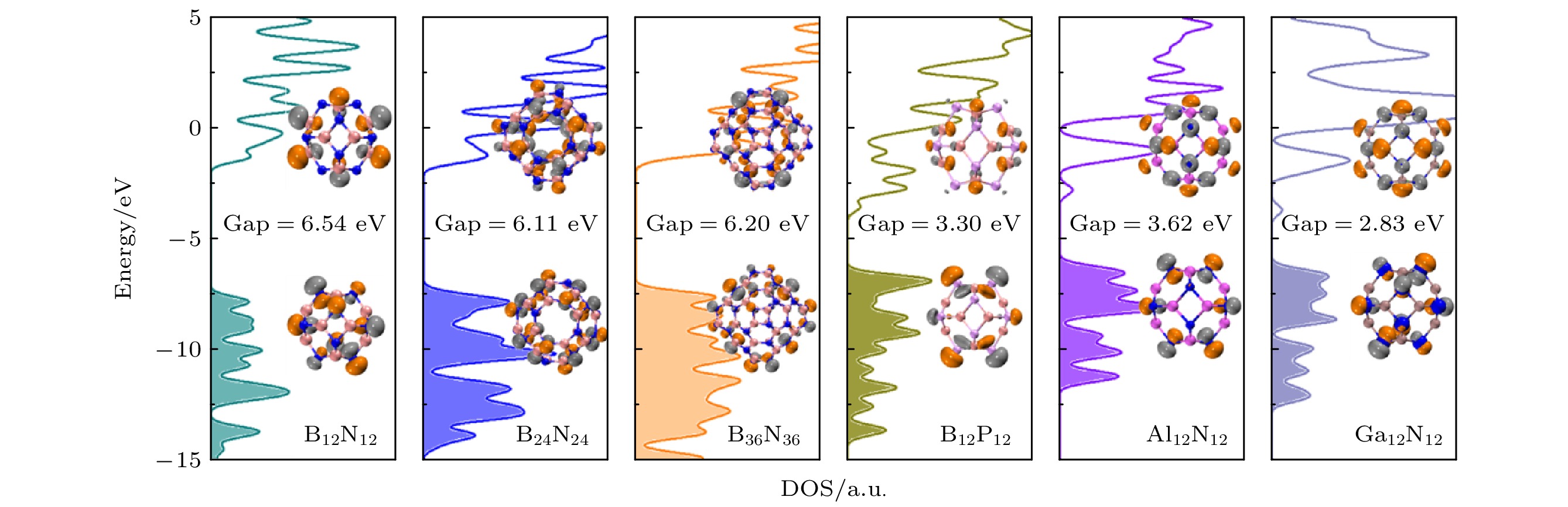
图 2 类富勒烯团簇的电子态密度. 填充和未填充区域分别代表占据态和未占据态, 数字给出HOMO-LUMO能隙, 插图为前线轨道分布, 等值面设为0.04 a.u.
Fig. 2. Density of states (DOS) of fullerene-like clusters in their ground states. The occupied states are shadowed. The HOMO-LUMO gap is given for each cluster. The insets display the HOMO and LUMO wavefunctions, with an isosurface value of 0.04 a.u.
图 4 (a)类富勒烯团簇的基态(
$ {E}_{\mathrm{H}\mathrm{L}}^{\mathrm{S}0} $ )、激发单重态($ {E}_{\mathrm{H}\mathrm{L}}^{\mathrm{S}1} $ )和三重态的HOMO-LUMO能隙($ {E}_{\mathrm{H}\mathrm{L}}^{\mathrm{T}1} $ ); (b)发射波长与$ {E}_{\mathrm{H}\mathrm{L}}^{\mathrm{S}1} $ 的关系图Fig. 4. (a) Histogram of HOMO-LUMO gaps of the ground state (
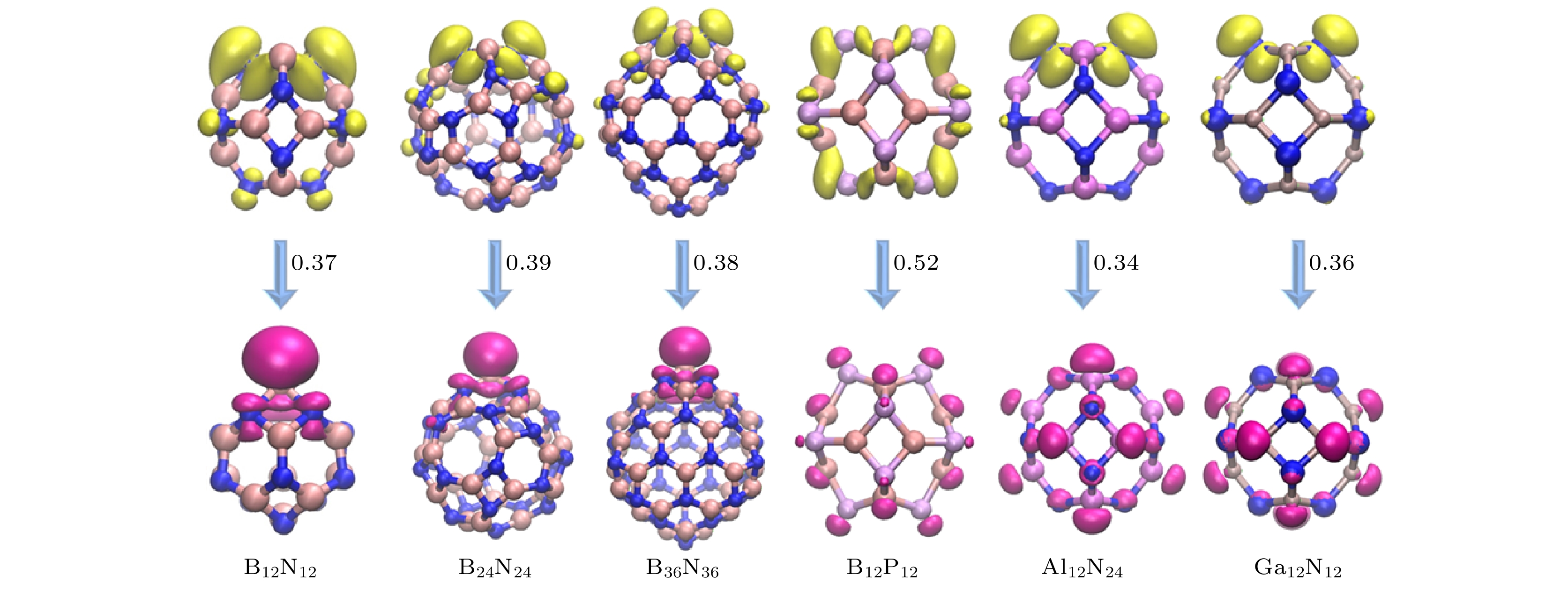
$ {E}_{\mathrm{H}\mathrm{L}}^{\mathrm{S}0} $ ), excited singlet state ($ {E}_{\mathrm{H}\mathrm{L}}^{\mathrm{S}1} $ ) and triplet state for the fullerene-like clusters ($ {E}_{\mathrm{H}\mathrm{L}}^{\mathrm{T}1} $ ); (b) relationship between emission wavelength and$ {E}_{\mathrm{H}\mathrm{L}}^{\mathrm{S}1} $ .图 5 类富勒烯团簇S1态的空穴(黄色)和电子(粉色)密度分布. 数字给出空穴和电子波函数的重叠值, 等值面设为0.04 a.u.
Fig. 5. Electron (magenta) and hole (yellow) density distributions for the excited singlet state of fullerene-like clusters. The overlap between hole and electron wavefunctions are given for each cluster, with an isosurface value of 0.04 a.u.
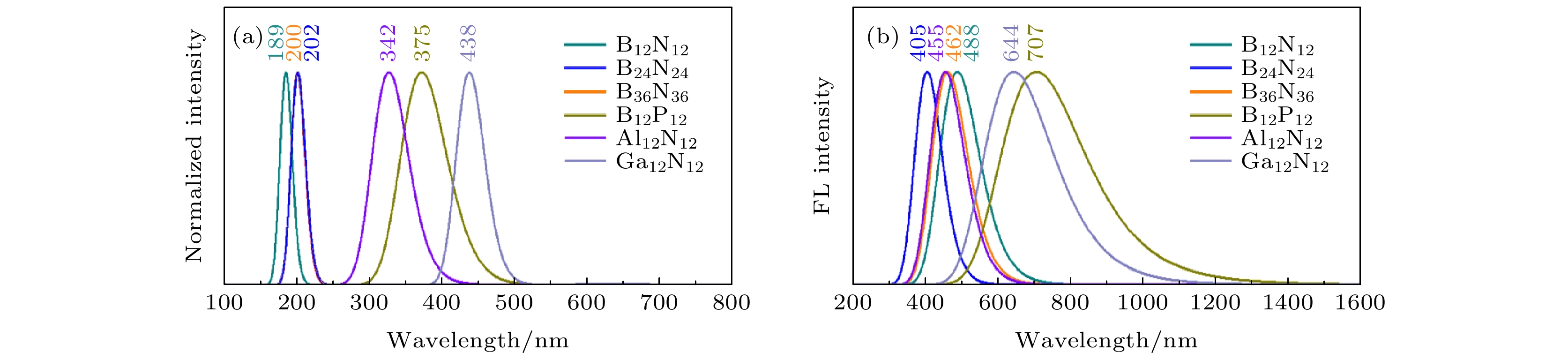
表 1 6种类富勒烯团簇的基态性质, 包括对称性(Sym.)、直径(D)、平均键长(d)、最小频率(νmin)、原子间电荷转移(CT)、键级(BO)、HOMO-LUMO能隙(
$ {E}_{\mathrm{H}\mathrm{L}}^{\mathrm{S}0} $ )以及吸收波长(λabs)Table 1. Ground-state properties of six fullerene-like clusters, including the symmetry (Sym.), diameter (D), average bond lengths (d), minimum frequency (νmin), Mulliken charge transfer (CT), average wiberg bond order (BO), HOMO-LUMO gap (
$ {E}_{\mathrm{H}\mathrm{L}}^{\mathrm{S}0} $ ), and absorption wavelength (λabs).Fullerene-like clusters Sym. D/Å d/Å νmin/
cm–1CT
/eBO $ {E}_{\mathrm{H}\mathrm{L}}^{\mathrm{S}0} $/eV λabs
/nmB12N12 Th 4.80 1.46 323.19 1.45 0.87 6.54 189.60 B24N24 S8 6.68 1.44 155.05 1.14 0.88 6.11 202.94 B36N36 Td 8.44 1.46 169.51 1.29 0.87 6.20 200.00 B12P12 Th 6.35 1.91 147.75 0.16 1.91 3.30 375.75 Al12N12 Th 5.96 1.82 154.33 1.93 1.83 3.62 342.54 Ga12N12 Th 6.17 1.88 104.80 1.76 0.62 2.83 438.16 表 2 6种类富勒烯团簇的激发态性质, 包括S1态的发射波长(λex)、HOMO-LUMO能隙(
$ {E}_{\mathrm{H}\mathrm{L}}^{\mathrm{S}1} $ )、激发能(ES1)、S1态和T1态的能量差(∆EST)、自旋-轨道耦合常数(V)、以及重组能(λS, λT)Table 2. Excited-state properties of six fullerene-like clusters, including emission wavelength (λex), HOMO-LUMO gap (
$ {E}_{\mathrm{H}\mathrm{L}}^{\mathrm{S}1} $ ), and emission energy of S1 state (ES1), energy difference between the S1 and T1 state (∆EST), spin-orbit coupling constant (V), and recombination energy of S1 state and T1 state (λS, λT).Fullerene-like clusters λex/nm $ {E}_{\mathrm{H}\mathrm{L}}^{\mathrm{S}1} $/eV ES1/eV ∆EST/eV V/cm–1 λS/eV λT/eV B12N12 488.38 3.02 2.54 0.25 0.70 7.0×10–3 3.0×10–4 B24N24 405.36 3.69 3.06 0.25 0.01 1.3×10–2 1.2×10–2 B36N36 462.40 3.32 2.68 0.27 0.26 8.0×10–3 1.0×10–2 B12P12 706.93 2.08 1.75 0.29 0.07 2.3×10–2 0.14 Al12N12 455.83 3.00 2.72 0.27 0.29 6.8×10–2 0.15 Ga12N12 644.47 2.19 1.92 0.14 9.86 1.9×10–2 3.0×10–3 -
[1] Baleizao C, Berberan-Santos M N 2008 Ann. N. Y. Acad. Sci. 1130 224
 Google Scholar
Google Scholar
[2] Dos Santos P L, Etherington M K, Monkman A P 2018 J. Mater. Chem. C 6 4842
 Google Scholar
Google Scholar
[3] Parker C A, Hatchard C G 1961 Trans. Faraday Soc. 57 1894
 Google Scholar
Google Scholar
[4] Lam S K, Lo D 1997 Chem. Phys. Lett. 281 35
 Google Scholar
Google Scholar
[5] Wolf M W, Legg K D, Brown R E, Singer L A, Parks J H 1975 J. Am. Chem. Soc. 97 4490
 Google Scholar
Google Scholar
[6] Maciejewski A, Szymanski M 1986 J. Phys. Chem. 90 6314
 Google Scholar
Google Scholar
[7] Yusa S, Kamachi M, Morishima Y 1998 Photochem. Photobiol. 67 519
 Google Scholar
Google Scholar
[8] Nickel B, Klemp D 1993 Chem. Phys. 174 297
 Google Scholar
Google Scholar
[9] Nickel B, Klemp D 1993 Chem. Phys. 174 319
 Google Scholar
Google Scholar
[10] Uoyama H, Goushi K, Shizu K, Nomura H, Adachi C 2012 Nature 492 234
 Google Scholar
Google Scholar
[11] Sato K, Shizu K, Yoshimura K, Kawada A, Miyazaki H, Adachi C 2013 Phys. Rev. Lett. 110 247410
[12] Tanaka H, Shizu K, Miyazaki H, Adachi C 2012 Chem. Commun. 48 11392
 Google Scholar
Google Scholar
[13] Lee J, Shizu K, Tanaka H, Nomura H, Yasuda T, Adachi C 2013 J. Mater. Chem. C 1 4599
 Google Scholar
Google Scholar
[14] Liu L, Wei Q, Cheng Y, Ma H, Xiong S, Zhang X 2020 J. Mater. Chem. C 8 5839
 Google Scholar
Google Scholar
[15] Bachilo S M, Benedetto A F, Weisman R B, Nossal J R, Billups W E 2000 J. Phys. Chem. A 104 11265
 Google Scholar
Google Scholar
[16] Arbohast J W, Foote C S 1991 J. Am. Chem. Soc. 113 8886
 Google Scholar
Google Scholar
[17] Wasielewski M R, O'Neil M P, Lykke K R, Pellin M J, Gruen D M 1991 J. Am. Chem. Soc. 113 2774
 Google Scholar
Google Scholar
[18] Argentine S M, Kota K T, Francis A H 1995 J. Am. Chem. Soc. 117 11762
 Google Scholar
Google Scholar
[19] Berberan-Santos M N, Garcia J M M 1996 J. Am. Chem. Soc. 118 9391
 Google Scholar
Google Scholar
[20] Wang Y 1992 J. Phys. Chem. 96 764
 Google Scholar
Google Scholar
[21] Salazar F A, Fedorov A, Berberan-Santos M N 1997 Chem. Phys. Lett. 271 361
 Google Scholar
Google Scholar
[22] Anthony S M, Bachilo S M, Weisman R B 2003 J. Phys. Chem. A 107 10674
 Google Scholar
Google Scholar
[23] Li X 2007 Acta Phys. -Chim. Sin. 23 1792
 Google Scholar
Google Scholar
[24] Augusto V, Baleizão C, Berberan-Santos M N, Farinha J P S 2010 J. Mater. Chem. 20 1192
 Google Scholar
Google Scholar
[25] Zhao J, Du Q, Zhou S, Kumar V 2020 Chem. Rev. 120 9021
 Google Scholar
Google Scholar
[26] Zhao J, Ma L, Tian D, Xie R 2008 J. Comput. Theor. Nanosci. 5 7
[27] Oku T, Nishiwaki A, Narita I 2004 Sci. Technol. Adv. Mater. 5 635
[28] Oku T, Nishiwaki A, Narita I, Gonda M 2003 Chem. Phys. Lett. 380 620
 Google Scholar
Google Scholar
[29] Oku T, Nishiwaki A, Narita I 2004 Solid State Commun. 130 171
 Google Scholar
Google Scholar
[30] Oku T, Narita I, Nishiwaki A 2004 J. Phys. Chem. Solids 65 369
 Google Scholar
Google Scholar
[31] Oku T, Kuno Masaki, Narita I 2002 Diamond Relat. Mater. 11 940
 Google Scholar
Google Scholar
[32] Fowler P W, Heine T, Mitchell D, Schmidt R, Seifert G 1996 J. Chem. Soc. , Faraday Trans. 92 2197
 Google Scholar
Google Scholar
[33] Oku T, Hirano T, Kuno M, Kusunose T, Niihara K, Suganuma K 2000 Mater. Sci. Eng. B 74 206
[34] Runge E, Gross E K U 1984 Phys. Rev. Lett. 52 997
 Google Scholar
Google Scholar
[35] Marques M A L, Gross E K U 2004 Annu. Rev. Phys. Chem. 55 427
 Google Scholar
Google Scholar
[36] Frisch M, Trucks G, Schlegel H, et al. 2016 Gaussian 16 (Revision A. 03) (Wallingford, CT: Gaussian. Inc.)
[37] Hongzhiwei Technology, Device Studio, Version 2021 A, China. 2021. Available online: https://iresearch.net.cn/cloudSoftware (accessed on 21 January 2022)
[38] Shuai Z 2020 Chin. J. Chem. 38 1223
 Google Scholar
Google Scholar
[39] Wu X, Liang X, Du Q, et al. 2018 J. Phys. Condens. Matter 30 354002
 Google Scholar
Google Scholar
[40] Wu X, Zhou S, Huang X, Chen M, Bruce King R, Zhao J 2018 J. Comput. Chem. 39 2268
 Google Scholar
Google Scholar
[41] Sai L, Wu X, Gao N, Zhao J, Bruce King R 2017 Nanoscale 9 13905
 Google Scholar
Google Scholar
[42] Wu X, Sai L, Zhou S, Zhou P, Chen M, Springborg M, Zhao J 2020 Phys. Chem. Chem. Phys. 22 12959
 Google Scholar
Google Scholar
[43] Watanabe K, Taniguchi T, Kanda H 2004 Nat. Mater. 3 404
 Google Scholar
Google Scholar
[44] Lambrecht W R L, Segall B 1993 Phys. Rev. B 47 9289
 Google Scholar
Google Scholar
[45] Zhu Q, Guo X, Zhang J 2019 J. Comput. Chem. 40 1578
 Google Scholar
Google Scholar
[46] Peng Q, Fan D, Duan R, Yi Y, Niu Y, Wang D, Shuai Z 2017 J. Phys. Chem. C 121 13448
 Google Scholar
Google Scholar
[47] Vincenzo S, Pagliai M, Ciabini L, Cardini G 2001 J. Phys. Chem. A 105 11192
 Google Scholar
Google Scholar
[48] Kumar K S, Patnaik A 2010 J. Comput. Chem. 31 1182
[49] Chen X K, Tsuchiya Y, Ishikawa Y, Zhong C, Adachi C, Brédas J L 2017 Adv. Mater. 29 1702767
 Google Scholar
Google Scholar
[50] Taffet E J, Olivier Y, Lam F, Beljonne D, Scholes G D 2018 J. Phys. Chem. Lett. 9 1620
 Google Scholar
Google Scholar
[51] Saigo M, Miyata K, Tanaka S, Nakanotani H, Adachi C, Onda K 2019 J. Phys. Chem. Lett. 10 2475
 Google Scholar
Google Scholar
[52] Kasha M 1950 Discuss. Faraday Soc. 9 14
 Google Scholar
Google Scholar
[53] Yuan F, Yuan T, Sui L, Wang Z, Xi Z, Li Y, Li X, Fan L, Tan Z, Chen A, Jin M, Yang S 2018 Nat. Commun. 9 1
 Google Scholar
Google Scholar
[54] Palit D K, Sapre A V, Mittal J P, Rao C N R 1992 Chem. Phys. Lett. 195 1
 Google Scholar
Google Scholar
[55] Sun Y P, Wang P, Hamilton N B 1993 J. Am. Chem. Soc. 115 6378
 Google Scholar
Google Scholar
[56] Khandelwal P, Poddar P 2017 J. Mater. Chem. B 5 9055
 Google Scholar
Google Scholar
[57] Marcus R A 1956 J. Chem. Phys. 24 966
 Google Scholar
Google Scholar
[58] Marcus R A Sutin N 1985 Biochim. Biophys. Acta 811 265
 Google Scholar
Google Scholar
[59] Marcus R A 1993 Rev. Mod. Phys. 65 599
 Google Scholar
Google Scholar
[60] Samanta P K, Kim D, Coropceanu V, Brédas J L 2017 J. Am. Chem. Soc. 139 4042
[61] Gao X, Bai S, Fazzi D, Niehaus T, Barbatti M, Thiel W 2017 J. Chem. Theory Comput. 13 515
 Google Scholar
Google Scholar
[62] Liu Y, Lin M, Zhao Y 2017 J. Phys. Chem. A 121 1145
[63] Turro, N J 1991 Modern Molecular Photochemistry (Sausalito, CA: University Science Books) p29
[64] Lu T, Chen F 2012 J. Comput. Chem. 33 580
 Google Scholar
Google Scholar
[65] Liu Z, Lu T, Chen Q 2020 Carbon 165 461
 Google Scholar
Google Scholar
[66] Grabowski Z R, Rotkiewicz K 2003 Chem. Rev. 103 3899
 Google Scholar
Google Scholar
计量
- 文章访问数: 9423
- PDF下载量: 186
- 被引次数: 0















 下载:
下载: