-
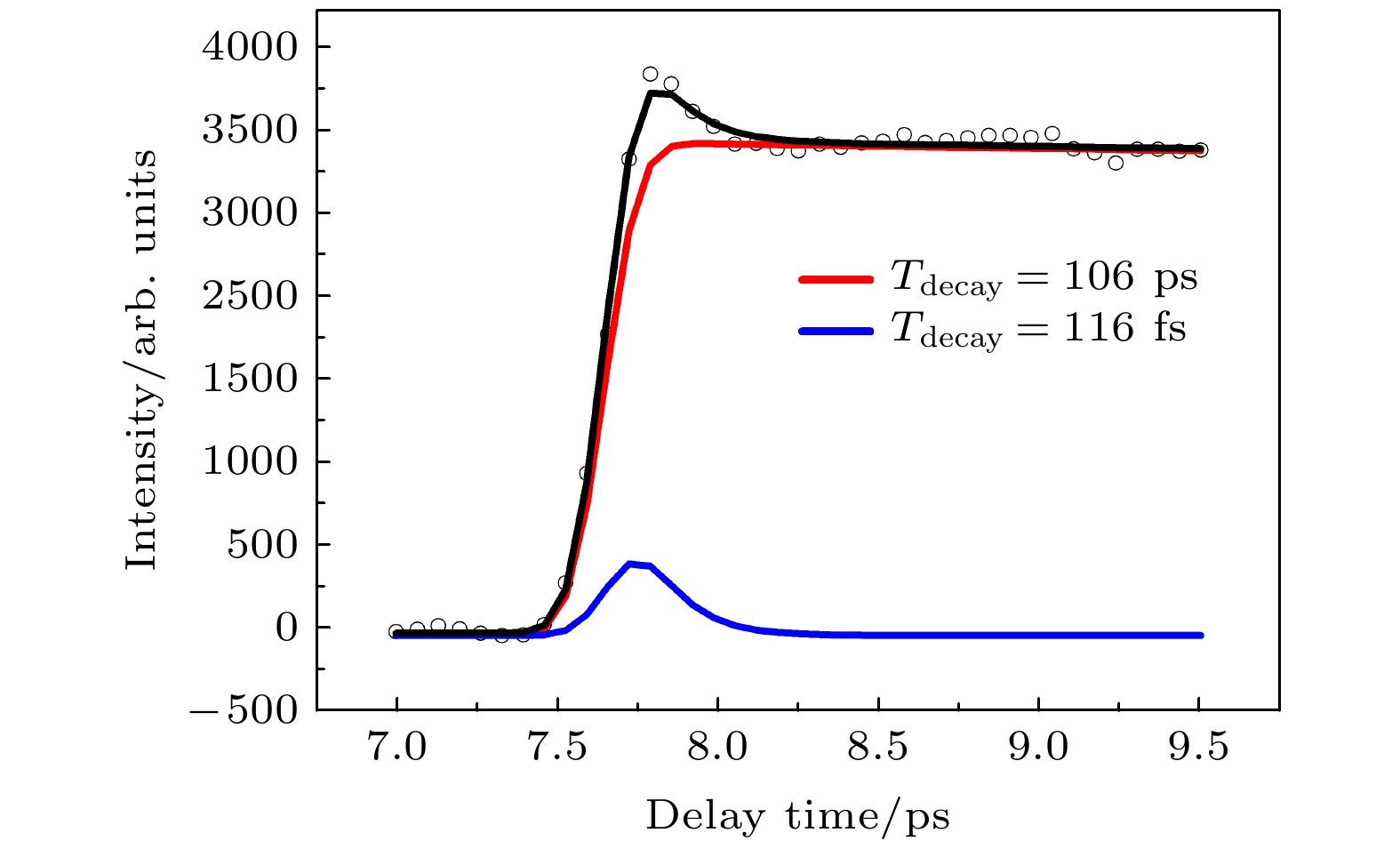
采用飞秒时间分辨质谱技术结合飞秒时间分辨光电子影像技术研究了苯乙炔分子电子激发态超快非绝热弛豫动力学. 用235 nm光作为泵浦光, 将苯乙炔分子激发到第二激发态S2, 用400 nm光探测激发态的演化过程. 时间分辨的母体离子的变化曲线用指数和高斯函数卷积得到不同的两个组分, 一个是超快衰减组分, 时间常数为116 fs, 一个是慢速组分, 时间常数为106 ps. 通过分析时间分辨的光电子影像得到光电子动能分布, 结合时间分辨光电子能谱数据发现, 时间常数为116 fs 的快速组分反映了S2态向S1态的内转换过程. 实验还表明S1态通过内转换被布局后向T1态的系间窜跃过程为重要的衰减通道. 本工作为苯乙炔分子S2态非绝热弛豫动力学提供了较清晰的物理图像.Interaction of light with matter has always been important in the field of natural science. Particularly, the ultrafast radiationless relaxation induced by UV light of molecular electronic excited states accompanied by ultrafast energy transfer plays an important role in the natural photophysical, photochemical and biological reactions. Generally, the molecular electronic excited state can be deactivated through a variety of decay channels, including dissociation, isomerization, internal conversion, intersysterm crossing, vibrational energy redistribution, and autoionization. This complexity of relaxation channels brings about a wide variety of deactivation mechanisms. The ultrafast nonadibatic relaxation dynamics of the excited state of phenylacetylene is studied by using femtosecond time-resolved photoelectron imaging and femtosecond time-resolved mass spectrometry. The first excited state S2 of phenylacetylene is excited by 235 nm pump light, and the excited state deactivation process is detected by 400 nm probe light. The time-dependent curves of parent ions include two exponential curves. One is the fast component with a time constant of 116 fs, and the other is the slow component with a time constant of 106 ps. The time-resolved photoelectron kinetic energy distribution is obtained from the time-resolved photoelectron images. Combined with the time-resolved photoelectron spectroscopy data, the fast component with a time constant of 116 fs is found to reflect the internal conversion process from S2 state to S1 state. The experimental results also show that S1 state is arranged by internal conversion, and the inter system jump process to T1 state is an important attenuation channel. This work provides a clearer physical picture for S1 state nonadibatic relaxation dynamics of phenylacetylene.
-
Keywords:
- phenylacetylene /
- photoelectron imaging /
- intersystem crossing /
- time-resolved spectroscopy
[1] Gruijl F R D 1999 Eur. J. Cancer. 35 2003
 Google Scholar
Google Scholar
[2] Ichihashi M, Ueda M, Budiyanto A, Bito T, Oka M, Fukunaga M, Tsuru K, Horikawa T 2003 Toxicology 189 21
 Google Scholar
Google Scholar
[3] Iqbal A, Stavros V G 2010 J. Phys. Chem. Lett. 1 227 4
 Google Scholar
Google Scholar
[4] Satzger H, Townsend D, Zgierski M Z, Patchkovskii S, Ullrich S, Stolow A 2006 P. Nati. Acad. Sci. 103 10196
 Google Scholar
Google Scholar
[5] Middleton C T, Harpe K D L, Su C, Law Y K, Carlos E C H, Kohler B 2009 Annu. Rev. Phys. Chem. 60 217
 Google Scholar
Google Scholar
[6] Zewail A H 2000 Angew. Chem. 39 2586
 Google Scholar
Google Scholar
[7] Schoenlein R W, Peteanu L A, Mathies R A, Shank C V 1991 Sci. 254 412
 Google Scholar
Google Scholar
[8] Gustavsson T, Improta R, Markovitsi D 2010 J. Phys. Chem. Lett 1 2453
 Google Scholar
Google Scholar
[9] Riedle E, Neusser H J, Schlag E W 1982 J. Phys. Chem. 86 4847
 Google Scholar
Google Scholar
[10] Otis C E, Knee J L, Johnson P M 1983 J. Phys. Chem. 87 2232
 Google Scholar
Google Scholar
[11] Toshinori S 2014 Bull. Chem. Soc. Jpn. 87 341
 Google Scholar
Google Scholar
[12] 蒿巧利 2017 博士学位论文 (武汉: 中国科学院武汉物理与数学研究所)
Hao Q L 2007 Ph. D. Dissertation (Wuhan: Institude of Physics and Mathematics, Chinese Academy of Sciences) (in Chinese)
[13] Farmanara P, Stert V, Radloff W, Hertel I V 2001 J. Phys. Chem. A 105 5613
 Google Scholar
Google Scholar
[14] Suzuki Y I, Horio T, Fuji T, Suzuki T 2011 J. Chem. Phys. 134 184313
 Google Scholar
Google Scholar
[15] Lee S H, Tang K C, Chen I C, et al. 2002 J. Phys. Chem. A 106 8979
 Google Scholar
Google Scholar
[16] Meisl M, Janoschek R 1986 J. Chem. Soc., Chem. Commun. 14 1066
 Google Scholar
Google Scholar
[17] Palmer I J, Ragazos I N, Bernardi F, Olivucci M, Robb M A 1993 J. Am. Chem. Soc. 115 673
 Google Scholar
Google Scholar
[18] Liu Y Z, Tang B F, Shen H, Zhang S, Zhang B 2010 Opt. Express. 18 5791
 Google Scholar
Google Scholar
[19] Wu G R, Hockett P, Stolow A 2011 Phys. Chem. Chem. Phys. 13 18447
 Google Scholar
Google Scholar
[20] 刘玉柱, Gerber Thomas, Knopp Gregor 2014 63 244208
 Google Scholar
Google Scholar
Liu Y Z, Thomas G, Gregor K 2014 Acta Phys. Sin. 63 244208
 Google Scholar
Google Scholar
[21] Suzuki T 2006 Annu. Rev. Phys. Chem. 57 555
 Google Scholar
Google Scholar
[22] 布玛丽亚·阿布力米提, 凌丰姿, 邓绪兰, 魏洁, 宋辛黎, 向梅, 张冰 2020 69 103301
 Google Scholar
Google Scholar
Abulimiti B, Ling F Z, Deng X L, Wei J, Song X L, Xiang M, Zhang B 2020 Acta Phys. Sin. 69 103301
 Google Scholar
Google Scholar
[23] Yan Y H, Long J Y, Liu Y Z 2020 Chem. Phys. 530 110611
 Google Scholar
Google Scholar
[24] 龙金友 2012博士学位论文(武汉: 中国科学院武汉物理与数学研究所)
Long J Y 2012 Ph. D. Dissertation (Wuhan: Institude of Physics and Mathematics, Chinese Academy of Sciences) (in Chinese)
[25] Leopold D G, Hemley R J, Vaida V 1981 J. Chem. Phys. 75 4758
 Google Scholar
Google Scholar
[26] Dribinski V, Ossadtchi A, Mandelshtam V A, Reisler H 2002 Rev. Sci. Instrum. 73 2634
 Google Scholar
Google Scholar
-
图 2 从Δt = 0 fs和Δt = 163 fs的影像中提取得到的光电子动能分布, 位于D0处的箭头表示(1+2')电离机制下最大的可资用能
Fig. 2. Photoelectron kinetic energy distributions at Δt = 0 ps and Δt = 92 ps. The arrow at D0 indicates the maximum electron energy by two-photon absorption of probe beam at 400 nm after one-photon excitation of pump at 235 nm.
-
[1] Gruijl F R D 1999 Eur. J. Cancer. 35 2003
 Google Scholar
Google Scholar
[2] Ichihashi M, Ueda M, Budiyanto A, Bito T, Oka M, Fukunaga M, Tsuru K, Horikawa T 2003 Toxicology 189 21
 Google Scholar
Google Scholar
[3] Iqbal A, Stavros V G 2010 J. Phys. Chem. Lett. 1 227 4
 Google Scholar
Google Scholar
[4] Satzger H, Townsend D, Zgierski M Z, Patchkovskii S, Ullrich S, Stolow A 2006 P. Nati. Acad. Sci. 103 10196
 Google Scholar
Google Scholar
[5] Middleton C T, Harpe K D L, Su C, Law Y K, Carlos E C H, Kohler B 2009 Annu. Rev. Phys. Chem. 60 217
 Google Scholar
Google Scholar
[6] Zewail A H 2000 Angew. Chem. 39 2586
 Google Scholar
Google Scholar
[7] Schoenlein R W, Peteanu L A, Mathies R A, Shank C V 1991 Sci. 254 412
 Google Scholar
Google Scholar
[8] Gustavsson T, Improta R, Markovitsi D 2010 J. Phys. Chem. Lett 1 2453
 Google Scholar
Google Scholar
[9] Riedle E, Neusser H J, Schlag E W 1982 J. Phys. Chem. 86 4847
 Google Scholar
Google Scholar
[10] Otis C E, Knee J L, Johnson P M 1983 J. Phys. Chem. 87 2232
 Google Scholar
Google Scholar
[11] Toshinori S 2014 Bull. Chem. Soc. Jpn. 87 341
 Google Scholar
Google Scholar
[12] 蒿巧利 2017 博士学位论文 (武汉: 中国科学院武汉物理与数学研究所)
Hao Q L 2007 Ph. D. Dissertation (Wuhan: Institude of Physics and Mathematics, Chinese Academy of Sciences) (in Chinese)
[13] Farmanara P, Stert V, Radloff W, Hertel I V 2001 J. Phys. Chem. A 105 5613
 Google Scholar
Google Scholar
[14] Suzuki Y I, Horio T, Fuji T, Suzuki T 2011 J. Chem. Phys. 134 184313
 Google Scholar
Google Scholar
[15] Lee S H, Tang K C, Chen I C, et al. 2002 J. Phys. Chem. A 106 8979
 Google Scholar
Google Scholar
[16] Meisl M, Janoschek R 1986 J. Chem. Soc., Chem. Commun. 14 1066
 Google Scholar
Google Scholar
[17] Palmer I J, Ragazos I N, Bernardi F, Olivucci M, Robb M A 1993 J. Am. Chem. Soc. 115 673
 Google Scholar
Google Scholar
[18] Liu Y Z, Tang B F, Shen H, Zhang S, Zhang B 2010 Opt. Express. 18 5791
 Google Scholar
Google Scholar
[19] Wu G R, Hockett P, Stolow A 2011 Phys. Chem. Chem. Phys. 13 18447
 Google Scholar
Google Scholar
[20] 刘玉柱, Gerber Thomas, Knopp Gregor 2014 63 244208
 Google Scholar
Google Scholar
Liu Y Z, Thomas G, Gregor K 2014 Acta Phys. Sin. 63 244208
 Google Scholar
Google Scholar
[21] Suzuki T 2006 Annu. Rev. Phys. Chem. 57 555
 Google Scholar
Google Scholar
[22] 布玛丽亚·阿布力米提, 凌丰姿, 邓绪兰, 魏洁, 宋辛黎, 向梅, 张冰 2020 69 103301
 Google Scholar
Google Scholar
Abulimiti B, Ling F Z, Deng X L, Wei J, Song X L, Xiang M, Zhang B 2020 Acta Phys. Sin. 69 103301
 Google Scholar
Google Scholar
[23] Yan Y H, Long J Y, Liu Y Z 2020 Chem. Phys. 530 110611
 Google Scholar
Google Scholar
[24] 龙金友 2012博士学位论文(武汉: 中国科学院武汉物理与数学研究所)
Long J Y 2012 Ph. D. Dissertation (Wuhan: Institude of Physics and Mathematics, Chinese Academy of Sciences) (in Chinese)
[25] Leopold D G, Hemley R J, Vaida V 1981 J. Chem. Phys. 75 4758
 Google Scholar
Google Scholar
[26] Dribinski V, Ossadtchi A, Mandelshtam V A, Reisler H 2002 Rev. Sci. Instrum. 73 2634
 Google Scholar
Google Scholar
计量
- 文章访问数: 8170
- PDF下载量: 98
- 被引次数: 0













 下载:
下载:



