-
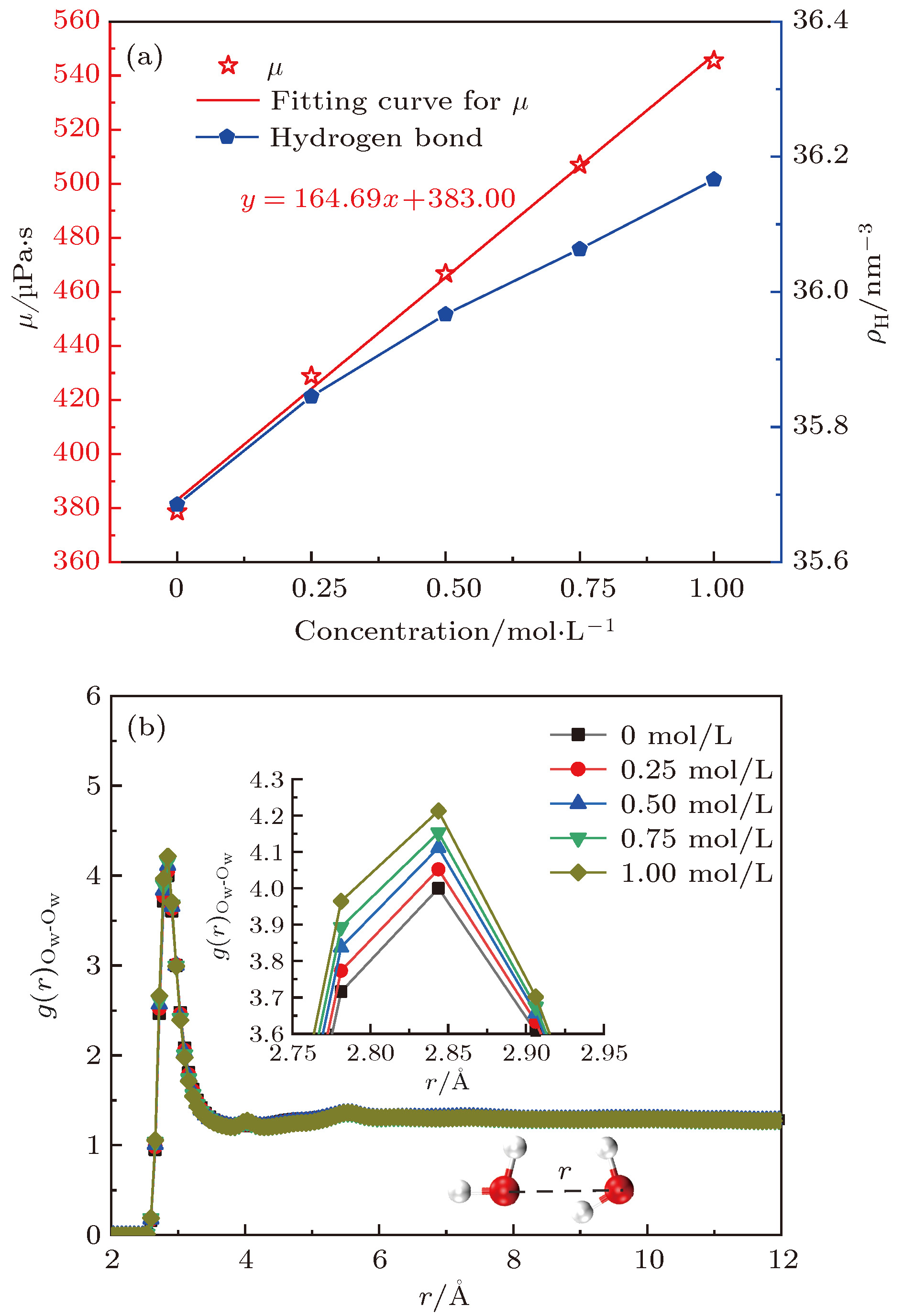
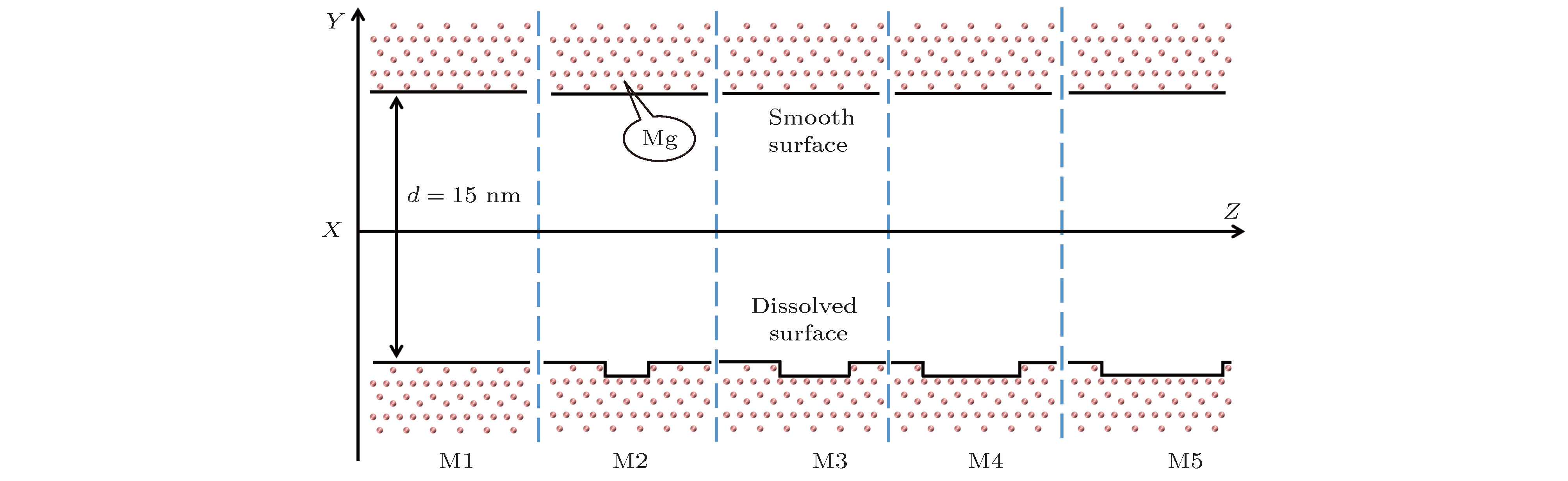
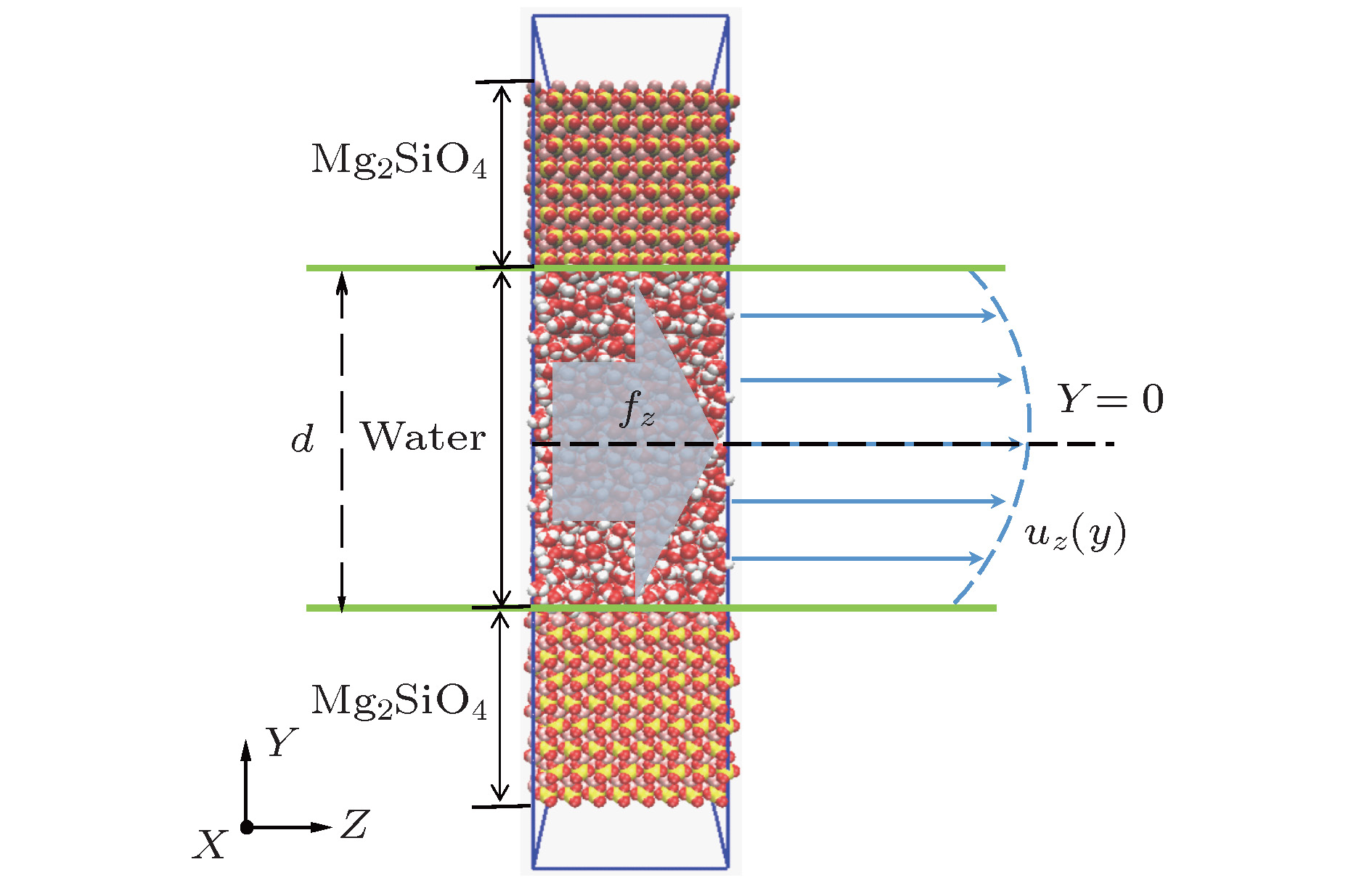
酸性环境引发的岩石孔隙表面溶解增加了孔隙内水溶液的盐离子浓度, 破坏了孔隙的表面结构. 本文采用分子动力学模拟的方法研究了纳米级岩石孔隙内水溶液的流动特性, 分析了盐离子浓度和孔隙表面结构对水流速度分布的影响及原因. 研究结果表明: 纳米级岩石孔隙内的水溶液流动符合泊肃叶流动特性, 流速呈“抛物线”分布; 随盐离子浓度增加, 水溶液内部氢键网络变得更为致密, 水黏度随其呈线性增长; 水溶液中离子浓度越大, 孔隙表面对水流动的阻力越大, 最大流速越小, 速度分布的“抛物线”曲率半径越大; 岩石孔隙表面结构的破坏改变了流动表面的粗糙程度, 增加了孔隙表面对H2O分子的吸引力. 随表面结构破坏程度的增大, 水溶液在近壁区域的密度增大, 流速降低; 当表面破坏程度达到50%时, 水溶液在近壁区域出现了明显的负边界滑移现象.The surface dissolution of rock nanopores, caused by the acidic environment, increases the salt concentration of water solution flowing in the nanopores, thereby destroying the surface structure of the rock, which can be found in CO2 geological sequestration and crude oil and shale gas exploration. In this paper, the molecular dynamics method is adopted to study the flow characteristics of water solution in the forsterite (Mg2SiO4) slit nanopores, by which the effects of salt concentration and structure destruction of pore surface on the velocity profiles of water solution confined in nanopores are systematically analyzed. The hydrogen bond density, radial distribution function (RDF) and water density distribution are calculated to explain the changes in viscosity, velocity profiles and interaction between water and nanopore surface. The results show that as the salt concentration increases, the water solution flow in the rock nanopore obeys the Hagen-Poiseuille equation, and the velocity profiles of water solution remain parabolic shape. However, the hydrogen bond network among water molecules becomes denser with salt concentration increasing, which can account for the linear increase in the viscosity of water solution. Besides, the higher salt concentration gives rise to the larger water flow resistance from the pore surface. As a result, with the salt concentration increasing, the maximum of water velocity decreases and the curvature radius of the parabolic velocity profile curve becomes bigger. Moreover, the surface structure destruction in rock nanopores changes the roughness of surface in the flow channel, which enhances the attraction of nanopore surface to H2O. As the structure destruction of nanopore surface deteriorates, the water density near the rough surface moves upward, whereas the velocity of water near the rough surface declines obviously. Interestingly, when the degree of surface structure destruction reaches 50%, a significant negative boundary slipping near the rough surface appears.
-
Keywords:
- rock nanopore /
- rock dissolution /
- molecular dynamics simulation /
- flow
[1] Schrag D P J 2007 Science 315 812
 Google Scholar
Google Scholar
[2] Liu B, Qi C, Zhao X, Teng G, Zhao L, Zheng H, Zhan K, Shi J 2018 J. Phys. Chem. C 122 26671
 Google Scholar
Google Scholar
[3] Cunningham A B, Gerlach R, Spangler L, Mitchell A C 2009 Energy Procedia. 1 3245
 Google Scholar
Google Scholar
[4] Pournik M, Nasr-El-Din H A, Mahmoud M A 2011 SPE Prod. Oper. 26 18
[5] Li Z, Xu Y, Yang L, Guo J, Chen J J 2016 Aust. J. Earth. Sci. 63 503
 Google Scholar
Google Scholar
[6] Black J R, Carroll S A, Haese R R 2015 Chem. Geol. 399 134
 Google Scholar
Google Scholar
[7] 黄桥高, 潘光, 宋保维 2014 63 054701
 Google Scholar
Google Scholar
Huang Q G, Pan G, Song B W 2014 Acta Phys. Sin. 63 054701
 Google Scholar
Google Scholar
[8] 葛宋, 陈民 2013 工程热 34 1527
Ge S, Chen M 2013 J. Eng. Therm. 34 1527
[9] 杨峰, 宁正福, 胡昌蓬, 王波, 彭凯, 刘慧卿 2013 石油学报 34 301
 Google Scholar
Google Scholar
Yang F, Ning Z F, Hu C P, Wang B, Peng K, Liu H Q 2013 Acta Petrol. Sin. 34 301
 Google Scholar
Google Scholar
[10] Eijkel J C, Van Den Berg A J M 2005 Microfluid. Nanofluid. 1 249
 Google Scholar
Google Scholar
[11] Karniadakis G, Beskok A, Aluru N 2006 Microflows and Nanoflows: Fundamentals and Simulation (Vol. 29) (Berlin: Springer Science & Business Media) pp13–15
[12] Wang S, Javadpour F, Feng Q H 2016 Fuel. 181 741
 Google Scholar
Google Scholar
[13] Ho T A, Striolo A 2015 AIChE J. 61 2993
 Google Scholar
Google Scholar
[14] Marcus Y J 2009 Chem. Rev. 109 1346
 Google Scholar
Google Scholar
[15] Ma J, Li K, Li Z, Qiu Y, Si W, Ge Y, Sha J, Liu L, Xie X, Yi H 2019 J. Am. Chem. Soc. 141 4264
 Google Scholar
Google Scholar
[16] van der Vegt N F, Haldrup K, Roke S, Zheng J, Lund M, Bakker H 2016 Chem. Rev. 116 7626
 Google Scholar
Google Scholar
[17] Aryal D, Ganesan V 2018 ACS Macro Lett. 7 739
 Google Scholar
Google Scholar
[18] 杨倩 2018 博士学位论文 (成都: 西南交通大学)
Yang Q 2018 Ph. D. Dissertation (Chengdu: Southwest Jiaotong University) (in Chinese)
[19] 张烨, 张冉, 常青, 李烨 2019 68 124702
 Google Scholar
Google Scholar
Zhang Y, Zhang R, Chang Q, Li H, 2019 Acta Phys. Sin. 68 124702
 Google Scholar
Google Scholar
[20] Rahmatipour H, Azimian A-R, Atlaschian O 2017 Physica A 465 159
 Google Scholar
Google Scholar
[21] 梅涛, 陈独秀, 杨历, 王坤, 苗瑞灿 2019 68 094701
 Google Scholar
Google Scholar
Mei T, Chen D X, Yang L, Wang K, Miao C C, 2019 Acta Phys. Sin. 68 094701
 Google Scholar
Google Scholar
[22] 南怡伶, 孔宪, 李继鹏, 卢滇楠 2017 化工学报 68 1786
Nan Y L, Kong X, Li J P, Lu D N 2017 J. Chem. Ind. Eng. (China) 68 1786
[23] 王胜, 徐进良, 张龙艳 2017 66 204704
 Google Scholar
Google Scholar
Wang S, Xu J L, Zhang L Y 2017 Acta Phys. Sin. 66 204704
 Google Scholar
Google Scholar
[24] 张冉, 谢文佳, 常青 2018 67 084701
 Google Scholar
Google Scholar
Zhang R, Xie W J, Chang Q 2018 Acta Phys. Sin. 67 084701
 Google Scholar
Google Scholar
[25] Markesteijn A, Hartkamp R, Luding S, Westerweel J 2012 J. Chem. Phys 136 134104
 Google Scholar
Google Scholar
[26] Yoshida H, Bocquet L 2016 J. Chem. Phys 144 234701
 Google Scholar
Google Scholar
[27] Xu J, Zhu C, Wang Y, Li H, Huang Y, Shen Y, Francisco J S, Zeng X C, Meng S 2019 Nano Res. 12 587
 Google Scholar
Google Scholar
[28] Nair R, Wu H, Jayaram P, Grigorieva I, Geim A 2012 Science 335 442
 Google Scholar
Google Scholar
[29] Huang H, Song Z, Wei N, Shi L, Mao Y, Ying Y, Sun L, Xu Z, Peng X 2013 Nat. Commun. 4 2979
 Google Scholar
Google Scholar
[30] Zhao L L, Ji J, Tao L, Lin S C 2016 Langmuir. 32 9188
 Google Scholar
Google Scholar
[31] Ross D J K, Bustin R M 2009 Mar. Pet. Geol. 26 916
 Google Scholar
Google Scholar
[32] Kerisit S, Weare J H, Felmy A R 2012 Geochim. Cosmochim. Acta 84 137
 Google Scholar
Google Scholar
[33] Wang J, Kalinichev A G, Kirkpatrick R J 2006 Geochim. Cosmochim. Acta 70 562
 Google Scholar
Google Scholar
[34] Cygan R T, Liang J-J, Kalinichev A G 2004 J. Phys. Chem. B. 108 1255
 Google Scholar
Google Scholar
[35] Yuet P K, Blankschtein D 2010 J. Phys. Chem. B 114 13786
 Google Scholar
Google Scholar
[36] Zhao L, Lin S, Mendenhall J D, Yuet P K, Blankschtein D 2011 J. Phys. Chem. B 115 6076
 Google Scholar
Google Scholar
[37] Verlet L 1967 Phys. Rev. 159 98
 Google Scholar
Google Scholar
[38] Delhommelle J, Philippe M 2001 Mol. Phys. 99 619
 Google Scholar
Google Scholar
[39] Darden T, York D, Pedersen L 1993 J. Chem. Phys. 98 10089
 Google Scholar
Google Scholar
[40] FrantzDale B, Plimpton S J, Shephard M S 2010 Eng. Comput. 26 205
 Google Scholar
Google Scholar
[41] Alvarez N J, Uguz A K 2013 Phys. Fluids 25 7336
[42] Span R, Wagner W J 1996 J. Phys. Chem. Ref. Data 25 1509
 Google Scholar
Google Scholar
[43] Liu L, Du J G, Zhao J J, Liu H, Gao H L, Chen Y X 2009 Phys. Earth Planet. 176 89
 Google Scholar
Google Scholar
-
图 3 (a)纯水和MgCl2含盐水中以0.32 nm半径的水合壳结构示意图; (b)不同MgCl2浓度下纳米级镁橄榄石孔隙内纯水和含盐水的+Z向速度分布
Fig. 3. (a) Snapshots for the solvation shell with a radius of 0.4 nm in pure water and MgCl2 solution, (b) the velocity profiles in the +Z direction of water solution in the forsterite nanopore with different MgCl2 concentrations.
-
[1] Schrag D P J 2007 Science 315 812
 Google Scholar
Google Scholar
[2] Liu B, Qi C, Zhao X, Teng G, Zhao L, Zheng H, Zhan K, Shi J 2018 J. Phys. Chem. C 122 26671
 Google Scholar
Google Scholar
[3] Cunningham A B, Gerlach R, Spangler L, Mitchell A C 2009 Energy Procedia. 1 3245
 Google Scholar
Google Scholar
[4] Pournik M, Nasr-El-Din H A, Mahmoud M A 2011 SPE Prod. Oper. 26 18
[5] Li Z, Xu Y, Yang L, Guo J, Chen J J 2016 Aust. J. Earth. Sci. 63 503
 Google Scholar
Google Scholar
[6] Black J R, Carroll S A, Haese R R 2015 Chem. Geol. 399 134
 Google Scholar
Google Scholar
[7] 黄桥高, 潘光, 宋保维 2014 63 054701
 Google Scholar
Google Scholar
Huang Q G, Pan G, Song B W 2014 Acta Phys. Sin. 63 054701
 Google Scholar
Google Scholar
[8] 葛宋, 陈民 2013 工程热 34 1527
Ge S, Chen M 2013 J. Eng. Therm. 34 1527
[9] 杨峰, 宁正福, 胡昌蓬, 王波, 彭凯, 刘慧卿 2013 石油学报 34 301
 Google Scholar
Google Scholar
Yang F, Ning Z F, Hu C P, Wang B, Peng K, Liu H Q 2013 Acta Petrol. Sin. 34 301
 Google Scholar
Google Scholar
[10] Eijkel J C, Van Den Berg A J M 2005 Microfluid. Nanofluid. 1 249
 Google Scholar
Google Scholar
[11] Karniadakis G, Beskok A, Aluru N 2006 Microflows and Nanoflows: Fundamentals and Simulation (Vol. 29) (Berlin: Springer Science & Business Media) pp13–15
[12] Wang S, Javadpour F, Feng Q H 2016 Fuel. 181 741
 Google Scholar
Google Scholar
[13] Ho T A, Striolo A 2015 AIChE J. 61 2993
 Google Scholar
Google Scholar
[14] Marcus Y J 2009 Chem. Rev. 109 1346
 Google Scholar
Google Scholar
[15] Ma J, Li K, Li Z, Qiu Y, Si W, Ge Y, Sha J, Liu L, Xie X, Yi H 2019 J. Am. Chem. Soc. 141 4264
 Google Scholar
Google Scholar
[16] van der Vegt N F, Haldrup K, Roke S, Zheng J, Lund M, Bakker H 2016 Chem. Rev. 116 7626
 Google Scholar
Google Scholar
[17] Aryal D, Ganesan V 2018 ACS Macro Lett. 7 739
 Google Scholar
Google Scholar
[18] 杨倩 2018 博士学位论文 (成都: 西南交通大学)
Yang Q 2018 Ph. D. Dissertation (Chengdu: Southwest Jiaotong University) (in Chinese)
[19] 张烨, 张冉, 常青, 李烨 2019 68 124702
 Google Scholar
Google Scholar
Zhang Y, Zhang R, Chang Q, Li H, 2019 Acta Phys. Sin. 68 124702
 Google Scholar
Google Scholar
[20] Rahmatipour H, Azimian A-R, Atlaschian O 2017 Physica A 465 159
 Google Scholar
Google Scholar
[21] 梅涛, 陈独秀, 杨历, 王坤, 苗瑞灿 2019 68 094701
 Google Scholar
Google Scholar
Mei T, Chen D X, Yang L, Wang K, Miao C C, 2019 Acta Phys. Sin. 68 094701
 Google Scholar
Google Scholar
[22] 南怡伶, 孔宪, 李继鹏, 卢滇楠 2017 化工学报 68 1786
Nan Y L, Kong X, Li J P, Lu D N 2017 J. Chem. Ind. Eng. (China) 68 1786
[23] 王胜, 徐进良, 张龙艳 2017 66 204704
 Google Scholar
Google Scholar
Wang S, Xu J L, Zhang L Y 2017 Acta Phys. Sin. 66 204704
 Google Scholar
Google Scholar
[24] 张冉, 谢文佳, 常青 2018 67 084701
 Google Scholar
Google Scholar
Zhang R, Xie W J, Chang Q 2018 Acta Phys. Sin. 67 084701
 Google Scholar
Google Scholar
[25] Markesteijn A, Hartkamp R, Luding S, Westerweel J 2012 J. Chem. Phys 136 134104
 Google Scholar
Google Scholar
[26] Yoshida H, Bocquet L 2016 J. Chem. Phys 144 234701
 Google Scholar
Google Scholar
[27] Xu J, Zhu C, Wang Y, Li H, Huang Y, Shen Y, Francisco J S, Zeng X C, Meng S 2019 Nano Res. 12 587
 Google Scholar
Google Scholar
[28] Nair R, Wu H, Jayaram P, Grigorieva I, Geim A 2012 Science 335 442
 Google Scholar
Google Scholar
[29] Huang H, Song Z, Wei N, Shi L, Mao Y, Ying Y, Sun L, Xu Z, Peng X 2013 Nat. Commun. 4 2979
 Google Scholar
Google Scholar
[30] Zhao L L, Ji J, Tao L, Lin S C 2016 Langmuir. 32 9188
 Google Scholar
Google Scholar
[31] Ross D J K, Bustin R M 2009 Mar. Pet. Geol. 26 916
 Google Scholar
Google Scholar
[32] Kerisit S, Weare J H, Felmy A R 2012 Geochim. Cosmochim. Acta 84 137
 Google Scholar
Google Scholar
[33] Wang J, Kalinichev A G, Kirkpatrick R J 2006 Geochim. Cosmochim. Acta 70 562
 Google Scholar
Google Scholar
[34] Cygan R T, Liang J-J, Kalinichev A G 2004 J. Phys. Chem. B. 108 1255
 Google Scholar
Google Scholar
[35] Yuet P K, Blankschtein D 2010 J. Phys. Chem. B 114 13786
 Google Scholar
Google Scholar
[36] Zhao L, Lin S, Mendenhall J D, Yuet P K, Blankschtein D 2011 J. Phys. Chem. B 115 6076
 Google Scholar
Google Scholar
[37] Verlet L 1967 Phys. Rev. 159 98
 Google Scholar
Google Scholar
[38] Delhommelle J, Philippe M 2001 Mol. Phys. 99 619
 Google Scholar
Google Scholar
[39] Darden T, York D, Pedersen L 1993 J. Chem. Phys. 98 10089
 Google Scholar
Google Scholar
[40] FrantzDale B, Plimpton S J, Shephard M S 2010 Eng. Comput. 26 205
 Google Scholar
Google Scholar
[41] Alvarez N J, Uguz A K 2013 Phys. Fluids 25 7336
[42] Span R, Wagner W J 1996 J. Phys. Chem. Ref. Data 25 1509
 Google Scholar
Google Scholar
[43] Liu L, Du J G, Zhao J J, Liu H, Gao H L, Chen Y X 2009 Phys. Earth Planet. 176 89
 Google Scholar
Google Scholar
计量
- 文章访问数: 6279
- PDF下载量: 92
- 被引次数: 0













 下载:
下载: