-
Cu-Ni-Si系铜合金有良好的导电、导热和机械性能, 被广泛用于电子元器件等领域. 设计Cu-Ni-Si系铜合金成分时, 析出相成分的确定是关键. 本文利用团簇加连接原子模型方法按“析出相”设计Cu-Ni-Si系铜合金的成分. 依据团簇选取准则, 选定 δ-Ni2Si, γ-Ni5Si2和β-Ni3Si相团簇式分别为 [Ni-Ni8Si5]Ni, [Si-Ni10]Si3和 [Si-Ni12]Si3; 在基体Cu含量原子分数为93.75%, 95%, 95.83%, 96.7% 和 97.5% 的每一成分点处, 分别按析出相δ-Ni2Si, γ-Ni5Si2和β-Ni3Si设计了系列Cu-Ni-Si合金的成分. 合金原料在充满氩气的真空电弧炉中熔炼成合金锭, 经 950 °C/1 h固溶水淬和 450 °C/4 h时效水淬处理. 当合金的导电性成为成分设计的主因时, 基体Cu含量分别在90%—95.63% 和95.63%—97.5% 成分区间时, 析出相分别按δ-Ni2Si和 γ-Ni5Si2设计; 基体Cu含量大于97.5%, 按δ-Ni2Si, γ-Ni5Si2或β-Ni3Si中任一相设计均可, 导电性基本没有差别. 如果合金的强度是成分设计的主因, 基体Cu含量分别在90%—93.93%, 93.93%—94.34%, 94.34%—95.63% 和95.63%—96.12% 成分区间时, 析出相对应于上述成分区间分别按δ-Ni2Si, γ-Ni5Si2, β-Ni3Si和 γ-Ni5Si2设计; 基体Cu含量一旦大于96.12%, 析出相按δ-Ni2Si, γ-Ni5Si2或β-Ni3Si中任一相设计均可.
-
关键词:
- Cu–Ni–Si合金 /
- 团簇加连接原子 /
- 相成分设计 /
- 高硬度 /
- 导电性
Cu-Ni-Si alloy has good electrical conductivity, thermal conductivity, high strength, and high hardness, and is widely used in electronic components and other fields. When the compositions of the Cu-Ni-Si alloy are designed, the determination of the phase component is critical. In this work, the composition of Cu-Ni-Si alloy is designed according to the "precipitation phase" by cluster-plus-glum-atom model. Following the cluster selection criteria, the δ-Ni2Si, γ-Ni5Si2 and β-Ni3Si phase clusters are determined, respectively, and the corresponding cluster formulas are [Ni-Ni8Si5]Ni,[Si-Ni10]Si3, and [Si-Ni12]Si3. the compositions of a series of Cu-Ni-Si alloys are designed according to the different precipitated phases of δ-Ni2Si, γ-Ni5Si2, and β-Ni3Si each with Cu atom content being 93.75%, 95%, 95.8%, 96.7% and 97.5%, respectively. The alloy raw material is melted into alloy ingot in an argon-filled vacuum arc furnace. The ingots undergoes solid-solution at 950 ° C for 1 hour and water quenching then aging treatment at 450 ° C for 4 hour and water quenching. The conductivity and Vickers hardness of the alloy are tested by conductivity meter and hardness meter, respectively. The microstructure of the alloy is characterized by x-ray diffraction (XRD), scanning electron microscopy (SEM), and transmission electron microscopy (TEM). In general, the electrical conductivity of Cu-Ni-Si is the main consideration in the design of alloy composition, the content values of matrix Cu atoms are in the ranges of 90%-95.63% and 95.63%-97.5% respectively, the precipitated phases are designed according to δ-Ni2Si and γ-Ni5Si2 respectively; the content of matrix Cu atoms is over 97.5%, it can be designed according to any phase of δ-Ni2Si, γ-Ni5Si2 and β-Ni3Si, with no difference in electrical conductivity among them. If the strength of the alloy is the main factor in the composition design, the content values of Cu atoms in the matrix are in the ranges of 90% — 93.93%, 93.93% — 94.34%, 94.34%— 95.63%, and 95.63%—96.12% respectively, according to the composition intervals the precipitated phases are designed as δ-Ni2Si, γ-Ni5Si2, β-Ni3Si, and γ-Ni5Si2, respectively. Once the content of Cu in the matrix is greater than 96.12%, the precipitated phase can be designed according to any of the phases of δ-Ni2Si, γ-Ni5Si2 and β-Ni3Si.-
Keywords:
- Cu–Ni–Si alloy /
- cluster plus glue atom /
- precipitation phase composition design /
- high hardness /
- electrical conductivity
[1] Gholami M, Vesely J, Altenberger I, Kuhn H A, Janecek M, Wollmann M, Wagner L 2017 J. Alloys Compd. 696 201
 Google Scholar
Google Scholar
[2] Li D M, Jiang B B, Li X N, Wang Qing, Dong C 2019 Acta Metall. Sinica DOI:10.11900/0412.1961.2019.00080
[3] Corson M G 1927 Aime. Trans. 43 5
[4] Corson M G 1927 Iron Age 119 421
[5] Okamoto M 1939 The II. Report. J. Jpn. Inst. Met. 3 336
 Google Scholar
Google Scholar
[6] Okamoto M 1939 The II. Report. J. Jpn. Inst. Met. 3 365
 Google Scholar
Google Scholar
[7] Robertson W D, Grenier E G, Nole V F 1961 Trans.: Met. Soc. Aime 221 503
[8] Lei Q, Lia Z, Wang M P, Zhang L, Gong S, Xiao Z, Pan Z Y 2011 J. Alloys Compd. 509 3617
 Google Scholar
Google Scholar
[9] Li D M, Wang Q, Jiang B B, Li X N, Zhou W L, Dong C, Wang H, Chen Q X 2017 PNSI 27 467 DOI:10.1016/j.pnsc.2017.06.006
[10] Lockyer S A, Noble F W 1994 J. Mater. Sci. 29 218
 Google Scholar
Google Scholar
[11] Futatsuka R 1997 J. Jpn. Copper Brass Res. Assoc. 36 25
[12] Zhao D M, Dong Q M, Liu P, Kang B X, Huang J L, Jin Z H 2003 Mater. Chem. Phys. 79 81
 Google Scholar
Google Scholar
[13] Yamamoto Y, Sasaki G, Odasin M 1999 J. Jpn Copper Brass Res. Assoc 38 204
[14] Ryu H J, Baik H K, Hong S H 2000 J. Mater. Sci. 35 3641
 Google Scholar
Google Scholar
[15] Zhao D M, Dong Q M, Liu P, Kang B X, Huang J L, Jin Z H 2003 Mater. Sci. Eng. A 361 93
 Google Scholar
Google Scholar
[16] Kim Y G, Seong T Y, Han J H 1986 J. Mater. Sci. 21 1357
 Google Scholar
Google Scholar
[17] Dong C, Wang Q, Qiang J B, Wang Y M, Jiang N, Han G, Li Y H, Wu J, Xia J H 2007 J. Phys. D: Appl. Phys. 40 273
 Google Scholar
Google Scholar
[18] 董闯, 董丹丹, 王清 2018 金属学报 54 293
 Google Scholar
Google Scholar
Dong C, Dong D D, Wang Q 2018 Acta Metall. Sin. 54 293
 Google Scholar
Google Scholar
[19] Pang C, Wang Q, Zhang R Q, Li Q, Dai X, Dong C, Liaw P K 2015 Mater. Sci. Eng., A 626 369
 Google Scholar
Google Scholar
[20] Wang Q, Ji C J, Wang Y M, Qiang J B, Dong C 2013 Metall. Mater. Trans. A 44 1872
 Google Scholar
Google Scholar
[21] 郝传璞, 王清, 马仁涛, 王英敏, 羌建兵, 董闯 2011 60 116101
 Google Scholar
Google Scholar
Hao C P, Wang Q, Ma R T, Wang Y M, Qiang J B, Dong C 2011 Acta Phys. Sin. 60 116101
 Google Scholar
Google Scholar
[22] Wang Z R, Qiang J B, Wang Y M, Wang Q, Dong D D, Dong C 2016 Acta Mater. 111 366
 Google Scholar
Google Scholar
[23] 姜贝贝, 王清, 董闯 2017 66 026100
Jiang B B, Wang Q, Dong C 2017 Acta Phys. Sin. 66 026100
[24] Hong H L, Wang Q, Dong C, Liaw P K 2014 Sci. Rep. 4 7065 DOI:10.1038/srep07065
[25] Takeuchi A, Inoue A 2005 Mater. Trans. 46 2817
 Google Scholar
Google Scholar
[26] Qian S N, Dong C, Liu T Y, Qin Y, Wang Q, Wu Y J, Gu L D, Zou J X, Heng X W, Peng L M, Zeng X Q 2018 J. Mater. Sci. Technol. 34 1132
 Google Scholar
Google Scholar
[27] Villars P 1997 Perason’s Handbook Copyright Materials (Park, OH:ASM Interational) pp1−2886
[28] 陈季香, 羌建兵, 王清, 董闯 2012 61 046102
 Google Scholar
Google Scholar
Chen J X, Qiang J B, Wang Q, Dong C 2012 Acta Phys. Sin. 61 046102
 Google Scholar
Google Scholar
[29] 王清 2005博士学位论文 (大连: 大连理工大学)
Wang Q 2005 Ph. D. Dissertation (Dalian: Dalian University of Technology) (in Chinese)
[30] Luo L J, Jiang W, Wang Q, Wang Y M, Han G, Dong C 2010 Philos. Mag. 90 3961
 Google Scholar
Google Scholar
[31] Hu T, Chen J H, Liu J Z, Liu Z R, Wu C L 2013 Acta Mater. 61 1210
 Google Scholar
Google Scholar
[32] Hu J, Shi Y N, Sauvage X, Sha G, Lu K 2017 Science 355 1292
 Google Scholar
Google Scholar
-
图 10 (a) Ni/Si分别为2, 2.5, 3时, 维氏硬度和导电性随CCu的变化; 三元相图中 (b) 导电性和(c)维氏硬度随Cu, Ni和Si元素的原子分数的变化***图(a)和(b)中均应为%IACS***
Fig. 10. (a) Ni/Si is 2, 2.5 and 3 respectively, the variation of vickers hardness and electrical conductivity as increase CCu; the variation of (b) electrical conductivity and (c) vickers hardness as atomic percent of Cu,Ni and Si in ternary phase diagram.
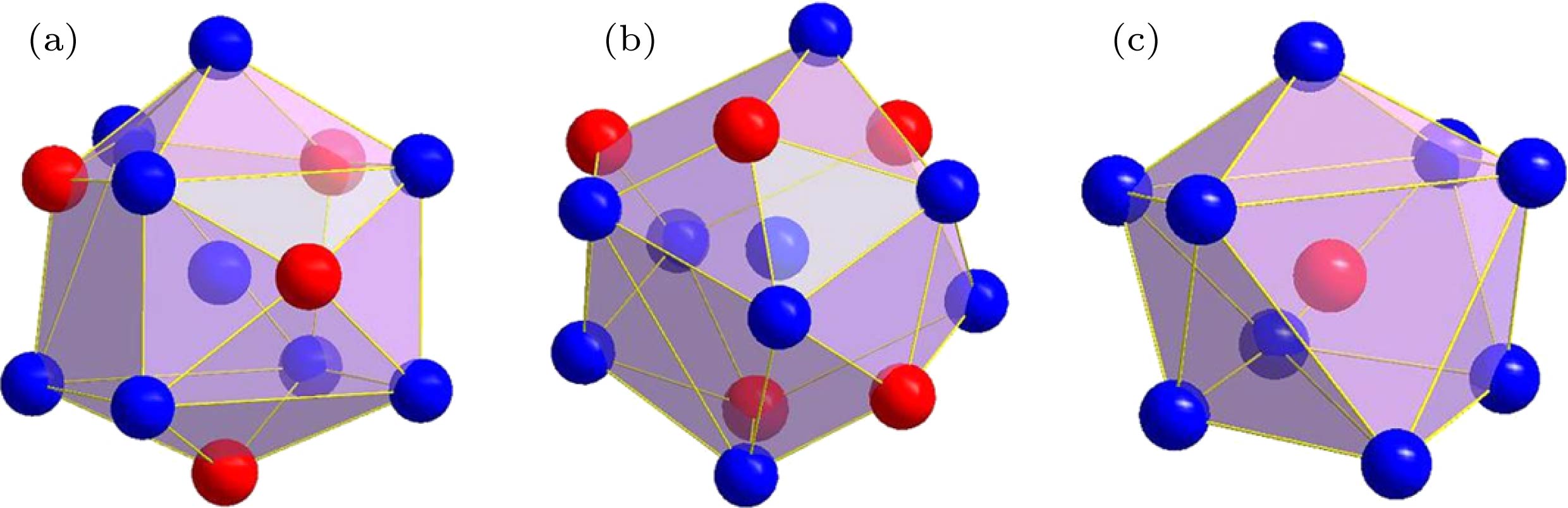
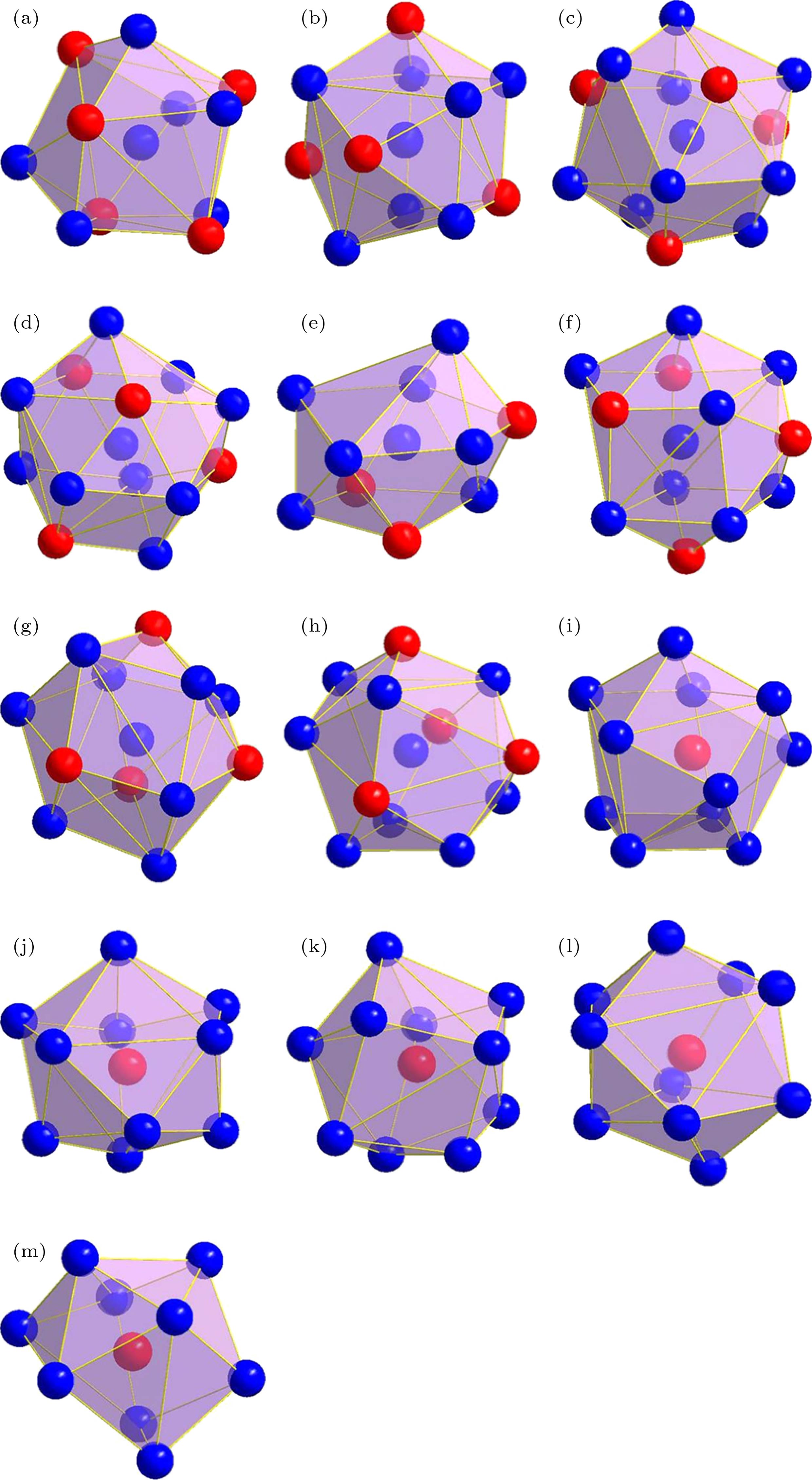
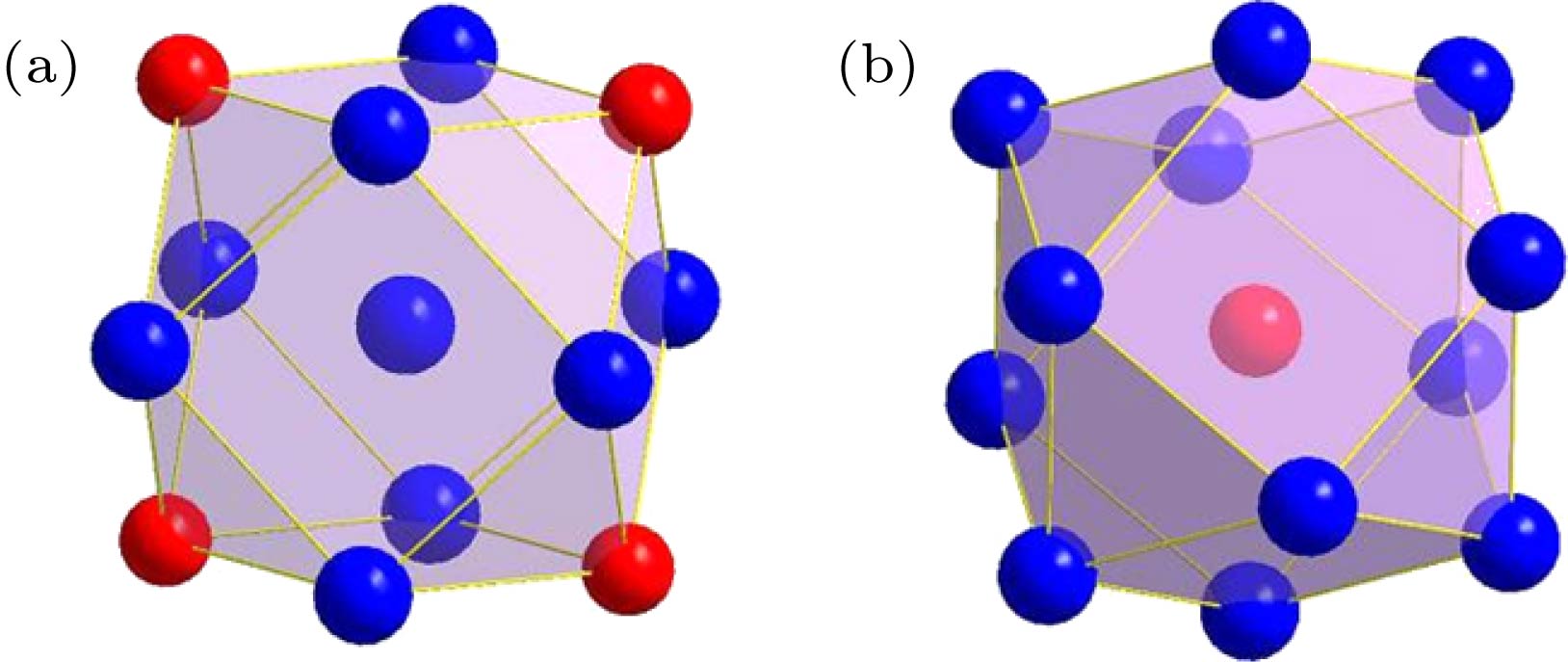
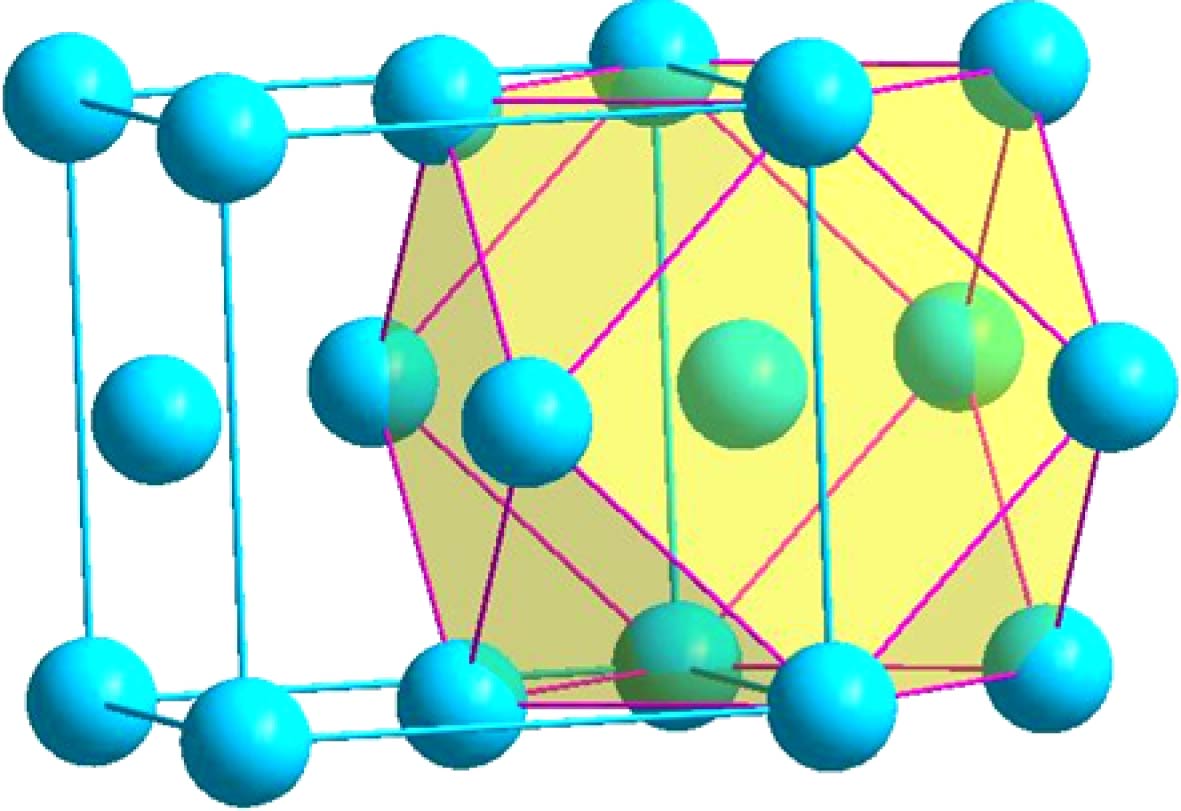
表 1 δ-Ni2Si相中以3种占位原子为心的径向原子分布
Table 1. Radial atomic distributions around 3 different sites in the δ-Ni2Si phase.
心部原子 壳层原子数目 壳层原子种类 壳层原子与心部原子的距离r/nm 径向原子密度ρ/nm-3 团簇 团簇构型 Ni1 1 Si1 0.20823 52.909 Ni9Si4 图2(a) 2 Si1 0.22614 82.615 1 Si1 0.23232 95.245 2 Ni2 0.25359 102.526 1 Ni2 0.26231 105.871 1 Ni2 0.26268 118.602 2 Ni1 0.27021 133.174 2 Ni2 0.27132 155.464 1 Si1 0.29611 128.795 Ni2 2 Si1 0.24629 47.964 Ni9Si5 图2(b) 2 Si1 0.24872 77.619 1 Si1 0.24972 92.092 2 Ni1 0.25359 117.172 2 Ni2 0.25818 138.792 1 Ni1 0.26231 145.573 1 Ni1 0.26268 158.136 2 Ni1 0.27132 167.423 1 Si1 0.32162 107.695 2 Ni2 0.34426 93.668 Si1 1 Ni1 0.20823 52.909 SiNi9 图2(c) 2 Ni1 0.22614 82.615 1 Ni1 0.23232 95.245 2 Ni2 0.24629 111.915 2 Ni2 0.24872 139.715 1 Ni2 0.24972 153.381 1 Ni1 0.29611 101.196 2 Si1 0.31484 99.496 1 Ni2 0.32162 100.515 2 Si1 0.34056 96.754 表 2 γ-Ni5Si2 相中以13种占位原子为心的径向原子分布
Table 2. Radial atomic distributions around 13 different sites in the γ-Ni5Si2 phase.
心部原子 壳层原子数目 壳层原子种类 壳层原子与心部原子的距离r/nm 径向原子密度ρ/nm–3 团簇 团簇构型 Ni1 3 Si5 0.23282 113.557 Ni7Si5 图4(a) 6 Ni8 0.25853 138.229 2 Si1 0.26156 160.177 6 Ni7 0.32954 120.138 6 Ni4 0.39376 93.896 6 Ni6 0.42716 91.935 3 Si5 0.43428 96.236 6 Ni8 0.45646 97.946 6 Si2 0.47401 100.921 2 Ni2 0.49734 91.258 Ni2 1 Ni2 0.23332 37.61 Ni8Si4 图4(b) 1 Si1 0.23578 54.668 3 Si4 0.2421 100.995 3 Ni6 0.24757 141.671 3 Ni5 0.25331 176.342 3 Ni5 0.34555 86.834 3 Ni7 0.34582 103.957 3 Ni3 0.38621 87.072 3 Si3 0.4174 78.829 3 Ni6 0.41824 88.149 3 Ni5 0.42043 96.421 3 Ni3 0.43617 94.99 Ni3 3 Si4 0.24409 65.696 Ni10Si4 图4(c) 1 Si2 0.24965 76.755 3 Ni5 0.25453 115.879 3 Ni6 0.26112 147.572 3 Ni5 0.26706 175.563 1 Si3 0.36558 73.329 3 Ni7 0.37339 82.588 3 Ni2 0.38621 87.072 3 Ni6 0.41335 81.169 3 Ni5 0.42222 85.68 Ni4 3 Si5 0.23225 76.265 Ni10Si4 图4(d) 1 Si3 0.25456 72.399 3 Ni8 0.25532 114.807 3 Ni7 0.25574 157.083 3 Ni8 0.27402 162.522 1 Si2 0.35821 77.949 3 Ni6 0.37974 78.514 3 Ni1 0.39376 82.159 3 Ni7 0.39797 90.948 3 Ni8 0.41739 88.689 Ni5 1 Si4 0.23142 38.544 Ni8Si3 图4(e) 1 Si3 0.23246 57.044 1 Si4 0.24177 67.606 1 Ni6 0.25071 75.786 1 Ni6 0.25316 88.328 1 Ni2 0.25331 102.866 1 Ni5 0.25375 116.951 1 Ni3 0.26706 137.942 1 Ni6 0.28031 130.136 2 Ni5 0.29349 132.276 Ni6 1 Si2 0.22997 39.278 Ni9Si4 图4(f) 1 Si1 0.23889 52.56 1 Si4 0.2445 65.366 1 Ni2 0.24757 78.706 1 Si3 0.24916 92.651 1 Ni5 0.25071 103.499 1 Ni5 0.25316 117.77 1 Ni8 0.25429 130.733 1 Ni7 0.25495 144.134 1 Ni7 0.26059 148.474 1 Ni3 0.26112 160.988 1 Ni7 0.26728 162.621 Ni7 1 Si3 0.22567 41.566 Ni9Si4 图4(g) 1 Si1 0.22958 59.218 1 Si5 0.23849 70.434 1 Si2 0.24038 85.982 1 Ni8 0.24588 96.408 1 Ni8 0.25044 106.443 1 Ni6 0.25495 115.307 1 Ni4 0.25574 128.522 1 Ni5 0.26021 135.569 1 Ni6 0.26059 148.474 1 Ni6 0.26728 150.111 1 Ni8 0.27224 153.893 Ni8 1 Si5 0.23059 38.962 Ni9Si4 图4(h) 1 Si5 0.24054 51.486 1 Ni8 0.2457 64.413 1 Ni7 0.24588 80.34 1 Si2 0.24656 95.613 1 Ni7 0.25044 106.443 1 Ni6 0.25429 116.207 1 Ni4 0.25531 129.173 1 Ni1 0.25853 138.229 Ni8 1 Si1 0.27197 130.605 Ni9Si4 图4(h) 1 Ni7 0.27224 142.055 1 Ni4 0.27402 150.913 Si1 3 Ni7 0.22958 78.957 SiNi11 图4(i) 1 Ni2 0.23578 91.113 3 Ni6 0.23889 140.161 1 Ni1 0.26156 120.132 3 Ni8 0.27197 142.479 3 Ni5 0.34489 87.334 3 Si5 0.35017 100.131 3 Si2 0.38543 87.602 3 Si3 0.39237 94.898 3 Si4 0.41136 92.647 Si2 3 Ni6 0.22997 78.556 SiNi10 图4(j) 3 Ni7 0.24038 120.375 3 Ni8 0.24656 159.354 1 Ni3 0.24965 168.861 3 Ni5 0.33462 89.249 3 Si5 0.35153 93.475 1 Ni4 0.35821 93.539 3 Si1 0.38543 87.602 3 Si3 0.38982 96.772 3 Si4 0.40727 95.466 Si3 3 Ni7 0.22567 83.132 SiNi10 图4(k) 3 Ni5 0.23246 133.102 3 Ni6 0.24916 154.418 1 Ni4 0.25456 159.277 3 Ni8 0.33473 89.161 3 Si4 0.35895 87.797 1 Ni3 0.36558 87.995 3 Si2 0.38982 84.676 3 Si1 0.39237 94.898 3 Si5 0.40056 100.344 Si4 2 Ni5 0.23142 57.816 SiNi10 图4(l) 2 Ni5 0.24177 84.507 2 Ni2 0.2421 117.827 2 Ni3 0.24409 147.817 2 Ni6 0.2445 179.758 2 Ni5 0.29512 120.803 2 Si3 0.35895 77.468 2 Si4 0.36743 81.857 4 Si4 0.39431 81.816 2 Si2 0.40727 81.323 Si5 2 Ni8 0.23059 58.364 SiNi9 图4(m) 2 Ni4 0.23225 95.331 1 Ni1 0.23282 113.559 2 Ni7 0.23849 140.868 2 Ni8 0.24054 171.621 2 Ni8 0.28808 119.887 2 Si1 0.35017 77.879 2 Si2 0.35153 76.979 4 Si5 0.37643 80.603 2 Si3 0.40056 74.329 表 3 β-Ni3Si相中以不同原子为心的径向原子分布
Table 3. Radial atomic distributions around 2 different sites in the β-Ni3Si phase.
表 4 Cu-Ni-Si-M (M = Fe or null)系列合金的Ni/Si(原子比)、团簇成分式、成分(原子分数)、维氏硬度(kgf/mm2)和导电率(%IACS)
Table 4. Ni/Si(at.%), Cluster formula, Composition(at.%), Vickers Hardness (kgf/mm2) and Electrical conductivity(%IACS) of Cu-Ni-Si-M (M = Fe or null) alloys.
Ni/Si (at.%) cluster formulas composition wt.% /at.% Vickers Hardness kgf·mm–2 Electrical Conductivity /%IACS 2 [(Fe1/15Ni9/15Si5/15)Cu12]Cu3 95.18Cu3.52Ni0.93Si0.37Fe
(Cu93.75Ni3.75Si2.08Fe0.42)258 35 ([(Ni10/15Si5/15)Cu12]Cu3)4+([CuCu12]Cu3) 96.14Cu3.11Ni0.75Si
(Cu95Ni3.33Si1.67)161 51 ([(Ni10/15Si5/15)Cu12]Cu3)2+([CuCu12]Cu3) 96.79Cu2.59Ni0.62Si
(Cu95.83Ni2.78Si1.39)189 35 {[(Ni10/15Si5/15)1.0602Cu12]Cu3}0.996 +{[CuCu12]Cu3} 97.4Cu2.1Ni0.5Si
(Cu96.7Ni2.2Si1.1)191 40 ([(Ni10/15Si5/15)Cu12]Cu3)2+([CuCu12]Cu3)3 98.08Cu1.55Ni0.37Si
(Cu97.5Ni1.67Si0.83)172 48 2.5 [(Fe1/14Ni9/14Si4/14)Cu12]Cu3 95.04Cu3.75Ni0.8Si0.41Fe
(Cu93.75Ni4.01Si1.79Fe0.45)262 32.5 ([(Ni10/14Si4/14)Cu12]Cu3)4+([CuCu12]Cu3) 96.03Cu3.33Ni0.64Si
(Cu95Ni3.57Si1.43)201 41 ([(Ni10/14Si4/14)Cu12]Cu3)2+([CuCu12]Cu3) 96.69Cu2.78Ni0.53Si
(Cu95.83Ni2.98Si1.19)201 38 {([(Ni10/14Si4/14) 1.0602Cu12]Cu3)}0.996 +([CuCu12]Cu3) 97.39Cu2.2Ni0.41Si
(Cu96.7Ni2.36Si0.94)168 41 ([Ni10/14Si4/14)Cu12]Cu3)2+([CuCu12]Cu3)3 98.02Cu1.66Ni0.32Si
(Cu97.5Ni1.79Si0.71)176 48 3 ([(Fe1/16Ni11/16Si4/16)Cu12]Cu3) 94.93Cu4.02Ni0.7Si0.35Fe
(Cu93.75Ni4.3Si1.56Fe0.39)241 30 ([(Ni12/16Si4/16)Cu12]Cu3)4+([CuCu12]Cu3) 95.94Cu3.5Ni0.56Si
(Cu95Ni3.75Si1.25)225 33 ([(Ni12/16Si4/16)Cu12]Cu3)2+([CuCu12]Cu3) 96.63Cu2.91Ni0.46Si
(Cu95.83Ni3.13Si1.04)191 36 {([(Ni12/16Si4/16)1.0602Cu12]Cu3)}0.996
+ ([CuCu12]Cu3)97.33Cu2.31Ni0.36Si
(Cu96.7Ni2.47Si0.83)160 39 ([(Ni12/16Si4/16)Cu12]Cu3)2+([CuCu12]Cu3)3 97.98Cu1.74Ni0.28Si
(Cu97.5Ni1.87Si0.63)171 47 -
[1] Gholami M, Vesely J, Altenberger I, Kuhn H A, Janecek M, Wollmann M, Wagner L 2017 J. Alloys Compd. 696 201
 Google Scholar
Google Scholar
[2] Li D M, Jiang B B, Li X N, Wang Qing, Dong C 2019 Acta Metall. Sinica DOI:10.11900/0412.1961.2019.00080
[3] Corson M G 1927 Aime. Trans. 43 5
[4] Corson M G 1927 Iron Age 119 421
[5] Okamoto M 1939 The II. Report. J. Jpn. Inst. Met. 3 336
 Google Scholar
Google Scholar
[6] Okamoto M 1939 The II. Report. J. Jpn. Inst. Met. 3 365
 Google Scholar
Google Scholar
[7] Robertson W D, Grenier E G, Nole V F 1961 Trans.: Met. Soc. Aime 221 503
[8] Lei Q, Lia Z, Wang M P, Zhang L, Gong S, Xiao Z, Pan Z Y 2011 J. Alloys Compd. 509 3617
 Google Scholar
Google Scholar
[9] Li D M, Wang Q, Jiang B B, Li X N, Zhou W L, Dong C, Wang H, Chen Q X 2017 PNSI 27 467 DOI:10.1016/j.pnsc.2017.06.006
[10] Lockyer S A, Noble F W 1994 J. Mater. Sci. 29 218
 Google Scholar
Google Scholar
[11] Futatsuka R 1997 J. Jpn. Copper Brass Res. Assoc. 36 25
[12] Zhao D M, Dong Q M, Liu P, Kang B X, Huang J L, Jin Z H 2003 Mater. Chem. Phys. 79 81
 Google Scholar
Google Scholar
[13] Yamamoto Y, Sasaki G, Odasin M 1999 J. Jpn Copper Brass Res. Assoc 38 204
[14] Ryu H J, Baik H K, Hong S H 2000 J. Mater. Sci. 35 3641
 Google Scholar
Google Scholar
[15] Zhao D M, Dong Q M, Liu P, Kang B X, Huang J L, Jin Z H 2003 Mater. Sci. Eng. A 361 93
 Google Scholar
Google Scholar
[16] Kim Y G, Seong T Y, Han J H 1986 J. Mater. Sci. 21 1357
 Google Scholar
Google Scholar
[17] Dong C, Wang Q, Qiang J B, Wang Y M, Jiang N, Han G, Li Y H, Wu J, Xia J H 2007 J. Phys. D: Appl. Phys. 40 273
 Google Scholar
Google Scholar
[18] 董闯, 董丹丹, 王清 2018 金属学报 54 293
 Google Scholar
Google Scholar
Dong C, Dong D D, Wang Q 2018 Acta Metall. Sin. 54 293
 Google Scholar
Google Scholar
[19] Pang C, Wang Q, Zhang R Q, Li Q, Dai X, Dong C, Liaw P K 2015 Mater. Sci. Eng., A 626 369
 Google Scholar
Google Scholar
[20] Wang Q, Ji C J, Wang Y M, Qiang J B, Dong C 2013 Metall. Mater. Trans. A 44 1872
 Google Scholar
Google Scholar
[21] 郝传璞, 王清, 马仁涛, 王英敏, 羌建兵, 董闯 2011 60 116101
 Google Scholar
Google Scholar
Hao C P, Wang Q, Ma R T, Wang Y M, Qiang J B, Dong C 2011 Acta Phys. Sin. 60 116101
 Google Scholar
Google Scholar
[22] Wang Z R, Qiang J B, Wang Y M, Wang Q, Dong D D, Dong C 2016 Acta Mater. 111 366
 Google Scholar
Google Scholar
[23] 姜贝贝, 王清, 董闯 2017 66 026100
Jiang B B, Wang Q, Dong C 2017 Acta Phys. Sin. 66 026100
[24] Hong H L, Wang Q, Dong C, Liaw P K 2014 Sci. Rep. 4 7065 DOI:10.1038/srep07065
[25] Takeuchi A, Inoue A 2005 Mater. Trans. 46 2817
 Google Scholar
Google Scholar
[26] Qian S N, Dong C, Liu T Y, Qin Y, Wang Q, Wu Y J, Gu L D, Zou J X, Heng X W, Peng L M, Zeng X Q 2018 J. Mater. Sci. Technol. 34 1132
 Google Scholar
Google Scholar
[27] Villars P 1997 Perason’s Handbook Copyright Materials (Park, OH:ASM Interational) pp1−2886
[28] 陈季香, 羌建兵, 王清, 董闯 2012 61 046102
 Google Scholar
Google Scholar
Chen J X, Qiang J B, Wang Q, Dong C 2012 Acta Phys. Sin. 61 046102
 Google Scholar
Google Scholar
[29] 王清 2005博士学位论文 (大连: 大连理工大学)
Wang Q 2005 Ph. D. Dissertation (Dalian: Dalian University of Technology) (in Chinese)
[30] Luo L J, Jiang W, Wang Q, Wang Y M, Han G, Dong C 2010 Philos. Mag. 90 3961
 Google Scholar
Google Scholar
[31] Hu T, Chen J H, Liu J Z, Liu Z R, Wu C L 2013 Acta Mater. 61 1210
 Google Scholar
Google Scholar
[32] Hu J, Shi Y N, Sauvage X, Sha G, Lu K 2017 Science 355 1292
 Google Scholar
Google Scholar
计量
- 文章访问数: 17059
- PDF下载量: 145
- 被引次数: 0













 下载:
下载: