-
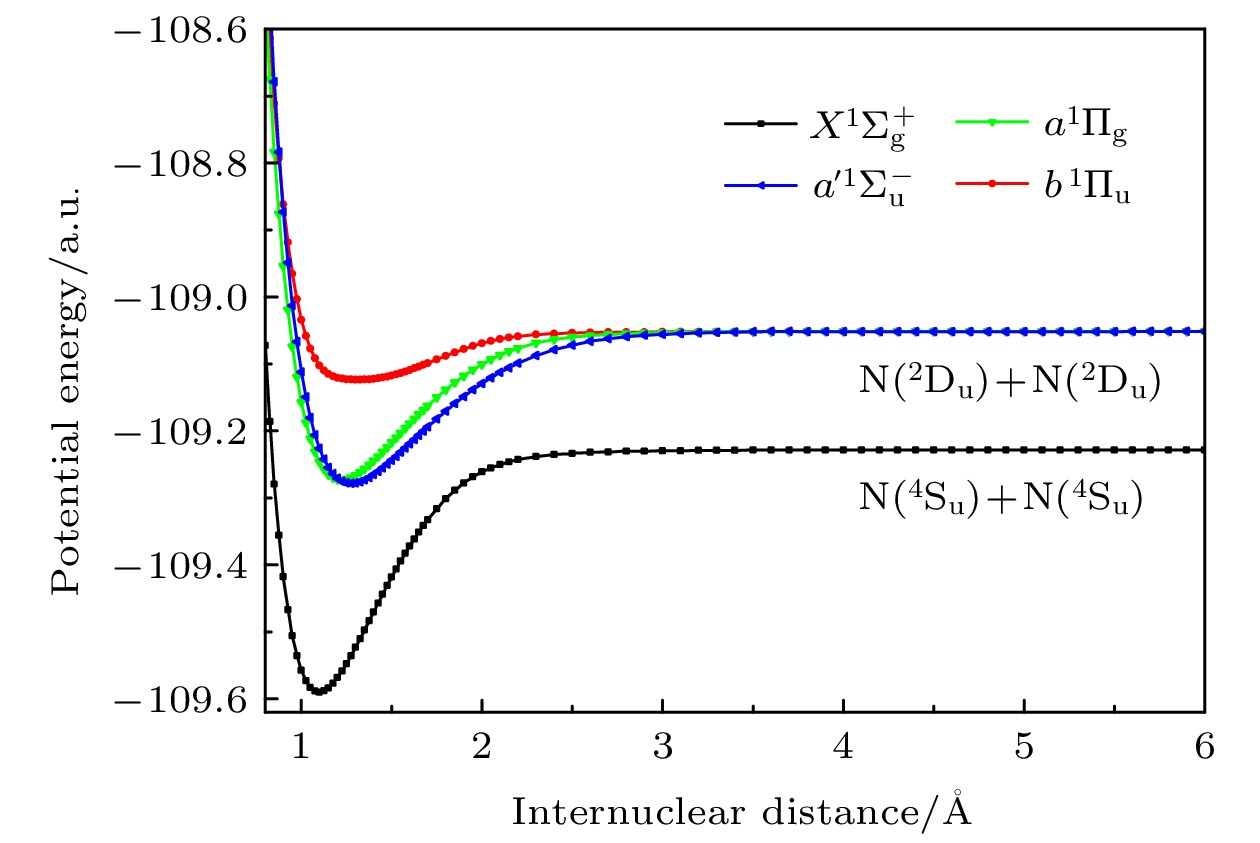
采用考虑Davidson修正的多参考组态相互作用(MRCI+Q)方法, 计算了氮气分子
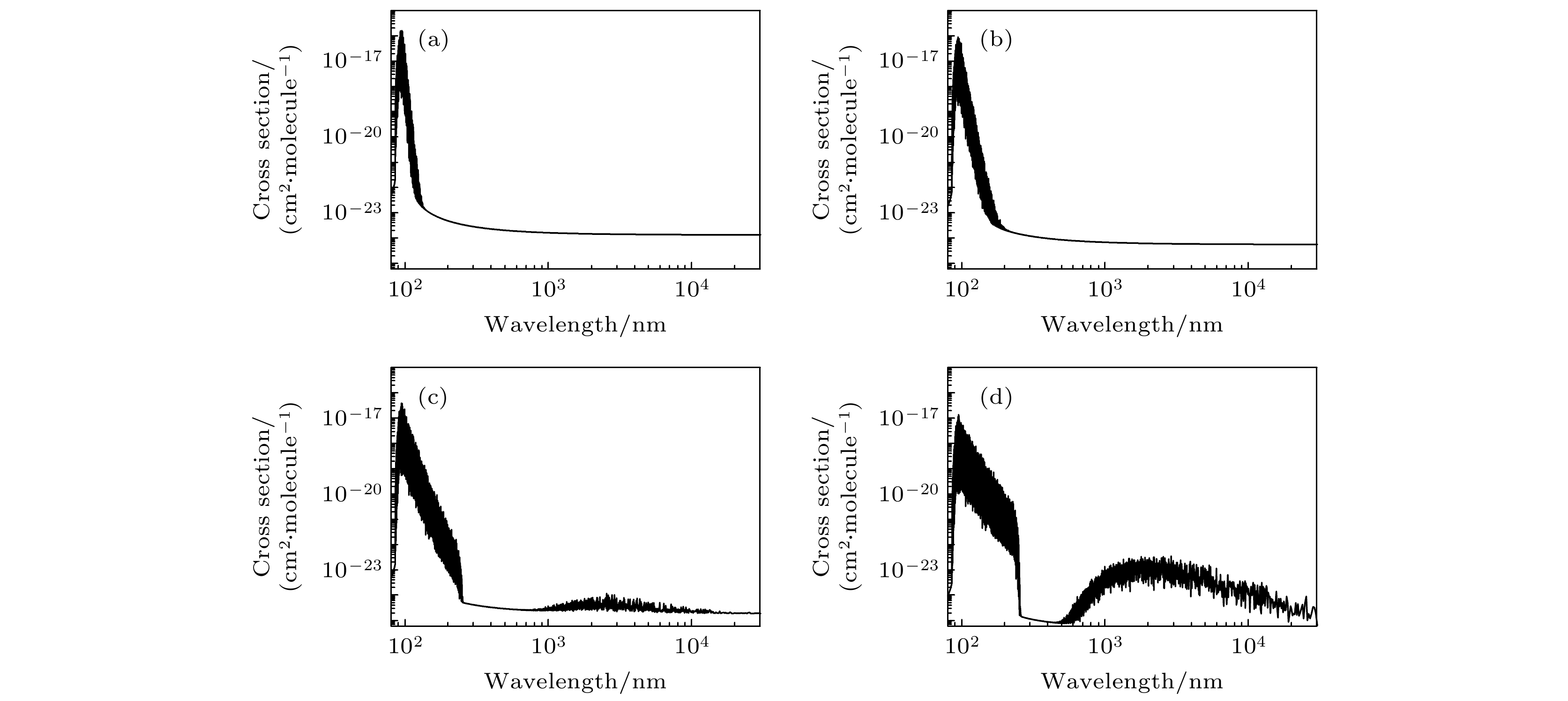
${X^1}\Sigma _{\rm{g}}^ + ,{a^\prime }^1\Sigma _{\rm{u}}^ - ,{a^1}{\Pi _{\rm{g}}}$ 和b1Пu电子态的势能曲线、偶极跃迁矩阵元、光谱常数和振动能级, 计算结果与其他实验和理论数据符合较好. 基于分子结构数据, 研究了氮气分子在100 atm (1 atm = 1.01×105 Pa)压强下, 295—20000 K温度范围内的不透明度. 结果表明, 在波长分布范围内, 不透明度随着温度的升高而变大; 当温度小于5000 K时, 不透明度主要分布在紫外区域, 当温度大于10000 K时, 激发态的贡献使得不透明度在红外区域也开始有明显的布居. 本文探明了温度效应对氮气分子不透明度的影响, 可以为天体物理和核武器领域提供理论和数据支持.Multi-reference configuration interaction (MRCI) approach with Davison size-extensivity correction (+Q) is employed to calculate the potential curves and dipole moments of${X^1}\Sigma _{\rm{g}}^ + ,{a^\prime }^1\Sigma _{\rm{u}}^ - ,{a^1}{\Pi _{\rm{g}}}$ and$b{}^1{\Pi _{\rm u}}$ electronic states of N2. The spectroscopic constants and vibrational level spaceings are calculated and in excellent agreement with the available theoretical results and experimental data. Based on the calculated molecular structure parameters, the opacities of N2 in a temperature range of 295–20000 K under a pressure of 100 atm (1 atm = 1.01×105 Pa) are presented. The results demonstrate that the wavelength range of absorption cross sections are enlarged with the temperature increasing. Moreover, the cross sections are mainly dominated in the range of ultraviolet for the cases with temperature T < 5000 K, while the obvious population can be found in the infrared ranges for the cases with temperature T > 10000 K due to the contribution of the excited states. The influence of temperature on the opacities of nitrogen molecule are investigated in the present work, which can provide theoretical and data support for researches of astrophysics and nuclear weapons.-
Keywords:
- nitrogen molecule /
- spectroscopic constants /
- opacities
[1] Lin X H, Liang G Y, Wang J G, Peng Y G, Shao B, Li R, Wu Y 2019 Chin. Phys. B 28 053101
 Google Scholar
Google Scholar
[2] Liang G Y, Peng Y G, Li R, Wu Y, Wang J G 2020 Chin. Phys. B 29 023101
 Google Scholar
Google Scholar
[3] Liang G Y, Peng Y G, Li R, Wu Y, Wang J G 2020 Chin. Phys. Lett. 37 123101
 Google Scholar
Google Scholar
[4] Li R, Liang G Y, Lin X H, Zhu Y H, Zhao S T, Wu Y 2019 Chin. Phys. B 28 043102
 Google Scholar
Google Scholar
[5] Xu X S, Dai A Q, Peng Y G, Wu Y, Wang J G 2018 J. Quant. Spectrosc. Radiat. Transfer 206 172
 Google Scholar
Google Scholar
[6] 马文, 靳奉涛, 袁建民 2007 56 5709
 Google Scholar
Google Scholar
Ma W, Jin F T, Yuan J M 2007 Acta Phys. Sin. 56 5709
 Google Scholar
Google Scholar
[7] Liu X M, Donald E S 2006 Astrophys. J. 645 1560
 Google Scholar
Google Scholar
[8] Bishop J, Feldman P D 2003 J. Geophys. Res. 108 1243
 Google Scholar
Google Scholar
[9] Strobel D F, Shemansky D E 1982 J. Geophys. Res. 87 1361
 Google Scholar
Google Scholar
[10] Stevens M H 2001 J. Geophys. Res. 106 3685
 Google Scholar
Google Scholar
[11] Vuitton V, Yelle R V, Anicich V G 2006 Astrophys. J. Lett. 647 L175
 Google Scholar
Google Scholar
[12] Liang M C, Heays A N, Lewis B R, Gibson S T, Yung Y L 2007 Astrophys. J. Lett. 664 L115
 Google Scholar
Google Scholar
[13] Knauth D C, Andersson B G, McCandliss S R, Moos H W 2004 Nature 429 636
 Google Scholar
Google Scholar
[14] Stark G, Huber K P, Yoshino K, Smith P L, Ito K 2005 J. Chem. Phys. 123 214303
 Google Scholar
Google Scholar
[15] Rothman L S, Jacquemart D, Barbe A, et al. 2005 J. Quant. Spectrosc. Radiat. Transfer 96 139
 Google Scholar
Google Scholar
[16] Rothman L S, Gordon I E, Barbe A, et al. 2009 J. Quant. Spectrosc. Radiat. Transfer 110 533
 Google Scholar
Google Scholar
[17] Gordon I E, Rothman L S, Hill C, et al. 2017 J. Quant. Spectrosc. Radiat. Transfer 203 3
 Google Scholar
Google Scholar
[18] Rothman L S, Wattson R B, Gamache R, Schroeder J W, McCann A 1995 Proc. Soc. 2471 105
 Google Scholar
Google Scholar
[19] Rothman L S, Gordon I E, Barber R J, Dothe H, Gamache R R, Goldman A, Perevalov V I, Tashkun S A and Tennyson J 2010 J. Quant. Spectrosc. Radiat. Transfer 111 2139
 Google Scholar
Google Scholar
[20] Lofthus A, Krupenie P H 1977 J. Phys. Chem. Ref. Data 6 113
 Google Scholar
Google Scholar
[21] Stark G, Smith P L, Huber K P, Yoshino K, Ito K 1992 J. Chem. Phys. 97 4809
 Google Scholar
Google Scholar
[22] Haverd V E, Lewis B R, Gibson S T, Stark G 2005 J. Chem. Phys. 123 214304
 Google Scholar
Google Scholar
[23] Robert Wu C Y, Judge D L, Matsui T 2006 J. Geophys. Res. 111 A5
 Google Scholar
Google Scholar
[24] Niu M L, Heays A N, Jones S, Salumbides E J, van Dishoeck E F, De Oliveira N, Nahon L, Ubachs W 2015 J. Mol. Spectrosc. 315 137
 Google Scholar
Google Scholar
[25] Heays A N, Lewis B R, De Oliveira N, Ubachs W 2019 J. Chem. Phys. 151 224305
 Google Scholar
Google Scholar
[26] Spelsberg D, Meyer W 2001 J. Chem. Phys. 115 6438
 Google Scholar
Google Scholar
[27] San-Fabián E, Pastor-Abia L 2003 Theor. Chem. Acc. 110 276
 Google Scholar
Google Scholar
[28] Hochlaf M, Ndome H, Hammoutène D, Vervloet M 2010 J. Phys. B: At. Mol. Opt. Phys. 43 245101
 Google Scholar
Google Scholar
[29] Shi D H, Xing W, Sun J F, Zhu Z L, Liu Y F 2012 Int. J. Quantum Chem. 112 1323
 Google Scholar
Google Scholar
[30] Xin Y, Ding H B 2014 Plasma Sci. Technol. 16 104
 Google Scholar
Google Scholar
[31] Lavín C, Velasco A M, Martín I 2010 Chem. Phys. Lett. 487 38
 Google Scholar
Google Scholar
[32] Lavín C, Velasco A M 2011 Astrophys. J. 739 16
 Google Scholar
Google Scholar
[33] Lavín C, Velasco A M 2016 Astrophys. J. 816 58
 Google Scholar
Google Scholar
[34] Lavín C, Velasco A M 2017 Astrophys. J. Suppl. Ser. 229 19
 Google Scholar
Google Scholar
[35] Velasco A M, Lavín C 2020 Astrophys. J. 899 57
 Google Scholar
Google Scholar
[36] Velasco A M, Alonso J L, Redondo P, Lavín C 2021 Astrophys. J. 922 100
 Google Scholar
Google Scholar
[37] Qin Z, Zhao J, Liu L 2019 Mol. Phys. 117 1
 Google Scholar
Google Scholar
[38] Liang R H, Liu Y M, Li F Y 2021 Phys. Scr. 96 125402
 Google Scholar
Google Scholar
[39] Weck P F, Schweitzer A, Kirby K, Hauschildt P H, Stancil P C 2004 Astrophys. J. 613 567
 Google Scholar
Google Scholar
[40] Werner H J, Knowles P J, Knizia G, et al. 2010 MOLPRO: a Package of ab initio Programs
[41] Le Roy R J 2002 LEVEL 7.5: a Computer Program for Solving the Radial Schrodinger Equation for Bound and Quasibound Levels (University of Waterloo, Chemical Physics Research Report CP-655)
[42] Langhoff S R, Davidson E R 1974 Int. J. Quantum. Chem. 8 61
 Google Scholar
Google Scholar
[43] Werner H J, Knowles P J 1985 J. Chem. Phys. 82 5053
 Google Scholar
Google Scholar
[44] Woon D E, Dunning T H 1995 J. Chem. Phys. 103 4572
 Google Scholar
Google Scholar
[45] Werner H J, Knowles P J 1988 J. Chem. Phys. 89 5803
 Google Scholar
Google Scholar
[46] Knowles P J, Werner H J 1988 Chem. Phys. Lett. 145 514
 Google Scholar
Google Scholar
[47] Moore C E 1975 Natl. Stand. Ref. Data Ser. (U.S. Natl. Bur. Stand) doc. 3 Sect. 5
[48] Müller T, Dallos M, Lischka H, Dubrovay Z, Szalay P G 2001 Theor. Chem. Acc. 105 227
 Google Scholar
Google Scholar
[49] Falzon C T, Chong D P, Wang F 2006 J. Comput. Chem. 27 163
 Google Scholar
Google Scholar
[50] Li X Z, Paldus J 2008 J. Chem. Phys. 129 54104
 Google Scholar
Google Scholar
[51] Huber H P, Herzberg G 1979 Molecular Spectra and Molecular Structure IV Constants of Diatomic Molecules (New York: Van Nostrand) p416
[52] Li X Z, Paldus J 2000 J. Chem. Phys. 113 9966
 Google Scholar
Google Scholar
[53] Li H, Le Roy R J 2007 J. Chem. Phys. 126 224301
 Google Scholar
Google Scholar
[54] Le Roy R J, Huang Y, Jary C 2006 J. Chem. Phys. 125 164310
 Google Scholar
Google Scholar
[55] Edwards S, Roncin J Y, Launay F, Rostas F 1993 J. Mol. Spectrosc. 162 257
 Google Scholar
Google Scholar
-
表 1 氮气分子的光谱常数
Table 1. Spectral constants of nitrogen molecular.
State Method Te/cm–1 ωe/cm–1 ωexe/cm–1 Be/cm–1 Re/Å De/eV ${X^1}\Sigma _{\rm{g}}^ + $ Present 0 2357.1168 14.3883 1.9968 1.0985 9.8396 MR-AQCC[48] 0 2337 1.1019 9.6426 MR-CISD[48] 0 2342 1.1016 9.6468 MR-CISD+Q[48] 0 2335 1.1019 9.6489 DFT(et-QZ3P-2D)[49] 0 2356 14.3 1.986 1.1012 DFT(ATZP) [49] 0 2346 13.3 1.974 1.1045 CCSD(T)[50] 0 2342.8 14.091 1.983 1.1014 CCSD[50] 0 2356.1 13.972 1.987 1.1003 CASSCF[30] 0 2358 1.092 9.82 Expt. [20] 0 2358.57 14.324 1.99824 1.09768 9.7593 $a'{}^1\Sigma _{\rm u}^ -$ Present 68344.098 1528.4544 11.4479 1.4794 1.2755 6.1725 MR-AQCC[48] 67762 1514 1.2807 6.1230 MR-CISD[48] 68480 1517 1.2804 6.0915 MR-CISD+Q[48] 67531 1513 1.2808 6.1254 DFT(et-QZ3P-2D) [49] 64968.9 1468 9.71 1.450 1.2887 DFT(ATZP) [49] 64578.2 1471 11.1 1.446 1.2906 MRCI[28] 69032 1523.6 11.91 1.4725 1.278 CASSCF[30] 1572 1.277 5.81 Expt. [20] 67739 1530.27 12.1 1.4801 1.2754 6.1278 a1Пg Present 69486.425 1691.4017 13.6099 1.6135 1.2215 6.04016 MR-AQCC[48] 69086 1676 1.2266 5.9587 MR-CISD[48] 69566 1691 1.2261 5.9568 MR-CISD+Q[48] 68951 1670 1.2268 5.9617 DFT(et-QZ3P-2D) [49] 69078.0 1684 12.4 1.609 1.2236 DFT(ATZP) [49] 68910.6 1647 14.0 1.601 1.2264 MRCI[28] 69971 1687.5 13.91 1.6034 1.225 CASSCF[30] 1676 1.230 6.30 Expt. [20] 68951.2 1694.2 13.9 1.6170 1.2203 5.9775 b1Пu Present 102357.2 682.0947 –5.9531 1.3929 1.319 1.9599 MR-AQCC[48] 101244 607 1.3456 1.9742 MR-CISD[48] 102333 632 1.3482 1.8942 MR-CISD+Q[48] 101018 600 1.3489 1.9859 MRCI[26] 101703.8 681.1 –8.8 1.437 Expt.[20] 100817.5 2.0265 Expt.[51] 101675 634.8 1.448 1.284 表 2 氮气分子
${X^1}\Sigma _{\rm{g}}^ + $ 态的振动能级间隔(Ev–Ev–1)(单位: cm–1)Table 2. Vibrational level spaceings (Ev–Ev–1) (in cm–1) for
${X^1}\Sigma _{\rm{g}}^ + $ state of nitrogen molecular.v Present 8R RMR CCSD[52] MR-AQCC[52] MR-ACPF[53] Expt.[54] Expt.[55] 1 2327.5 2336.5 2330.41 2328.54 2329.9 2329.9 2 2299.4 2308.4 2301.81 2299.89 2301.3 2301.2 3 2270.5 2279.8 2273.14 2271.18 2272.5 2272.6 4 2242.0 2251.4 2244.47 2242.45 2243.8 2243.8 5 2213.1 2222.5 2215.74 2213.69 2215.1 2215.0 6 2184.4 2193.7 2186.98 2184.87 2186.2 2186.2 7 2155.6 2164.7 2158.17 2156.01 2157.4 2157.4 8 2126.6 2135.3 2129.31 2127.10 2128.4 2128.4 9 2097.6 2106.0 2100.40 2098.13 2099.5 2099.5 10 2068.7 2076.4 2071.43 2069.09 2070.4 2070.4 11 2039.6 2046.7 2042.39 2040.02 2041.4 2041.4 12 2010.3 2016.8 2013.29 2010.84 2012.1 2012.1 13 1981.1 1987.0 1984.10 1981.58 1982.9 1983.0 14 1951.7 1956.9 1954.83 1952.26 1953.6 1953.5 15 1922.2 1927.0 1925.43 1922.79 1924.1 1924.2 16 1892.7 1896.9 1895.96 1893.25 1894.6 1894.7 17 1863.1 1866.7 1866.31 1863.53 1864.9 1865.1 18 1833.6 1836.6 1836.55 1833.69 1835.0 1835.4 19 1803.8 1806.3 1806.60 1803.68 1805.0 1805.6 20 1773.6 1775.9 1774.6 1775.6 21 1743.3 1745.5 1744.1 1745.7 22 1712.7 1714.8 1713.3 1715.5 23 1681.8 1684.0 1682.1 1685.0 24 1650.5 1652.8 1650.5 1655.0 25 1618.8 1621.6 1618.4 1624.0 26 1586.5 1585.9 27 1553.8 1552.8 28 1520.8 1519.0 29 1487.3 1484.7 30 1453.2 表 3 氮气分子
$ a'{}^1\Sigma _{\rm u}^ - $ ,$ a{}^1{\Pi _{\rm g}} $ 和$ b{}^1{\Pi _{\rm u}} $ 态的振动能级间隔(Ev – Ev–1)(单位: cm–1)Table 3. Vibrational level spaceings (Ev – Ev–1) (in cm–1) for
$a'{}^1\Sigma _{\rm u}^ -$ ,$ a{}^1{\Pi _{\rm g}} $ and$ b{}^1{\Pi _{\rm u}} $ states of nitrogen molecular.v $ { {a} }'{}^1\Sigma _{ {\rm u} }^ - $ a1Пg b1Пu Present Expt.[20] Present Expt.[20] Present Expt.[20] 1 1506.7 1506.24 1664.6 1666.34 645.2 645.4 2 1482.8 1482.45 1637.6 1638.51 710.9 705.3 3 1459.3 1458.90 1609.6 1610.77 745.2 747.6 4 1436.0 1435.57 1581.8 1583.07 763.7 774.8 5 1412.8 1412.47 1554.5 1555.46 772.9 789.6 6 1389.8 1389.58 1527.3 1527.93 776.4 794.4 7 1367.4 1366.88 1500.1 1500.49 774.8 791.4 8 1345.0 1344.41 1473.3 1473.15 770.5 782.8 9 1322.8 1322.10 1446.4 1445.91 762.7 770.2 10 1300.7 1300.00 1419.7 1418.77 752.4 754.8 11 1278.8 1278.06 1393.1 1391.77 740.2 737.9 12 1257.1 1256.31 1366.6 1364.87 725.2 719.8 13 1235.8 1234.70 1340.3 1338.12 708.4 701.0 14 1214.5 1213.27 1314.2 1311.50 689.0 681.4 15 1193.1 1191.98 1288.2 1285.03 667.1 660.5 16 1172.0 1170.84 1262.1 642.1 637.9 17 1151.2 1149.83 1236.4 613.4 612.6 18 1130.4 1128.95 1210.9 580.3 584.0 19 1109.6 1108.19 1185.3 541.8 551.0 20 1088.8 1159.7 21 1068.1 1134.0 22 1047.5 1108.4 23 1026.9 1082.8 24 1006.2 1057.1 25 985.4 1031.2 26 964.5 1005.0 27 943.4 978.4 28 922.3 951.4 -
[1] Lin X H, Liang G Y, Wang J G, Peng Y G, Shao B, Li R, Wu Y 2019 Chin. Phys. B 28 053101
 Google Scholar
Google Scholar
[2] Liang G Y, Peng Y G, Li R, Wu Y, Wang J G 2020 Chin. Phys. B 29 023101
 Google Scholar
Google Scholar
[3] Liang G Y, Peng Y G, Li R, Wu Y, Wang J G 2020 Chin. Phys. Lett. 37 123101
 Google Scholar
Google Scholar
[4] Li R, Liang G Y, Lin X H, Zhu Y H, Zhao S T, Wu Y 2019 Chin. Phys. B 28 043102
 Google Scholar
Google Scholar
[5] Xu X S, Dai A Q, Peng Y G, Wu Y, Wang J G 2018 J. Quant. Spectrosc. Radiat. Transfer 206 172
 Google Scholar
Google Scholar
[6] 马文, 靳奉涛, 袁建民 2007 56 5709
 Google Scholar
Google Scholar
Ma W, Jin F T, Yuan J M 2007 Acta Phys. Sin. 56 5709
 Google Scholar
Google Scholar
[7] Liu X M, Donald E S 2006 Astrophys. J. 645 1560
 Google Scholar
Google Scholar
[8] Bishop J, Feldman P D 2003 J. Geophys. Res. 108 1243
 Google Scholar
Google Scholar
[9] Strobel D F, Shemansky D E 1982 J. Geophys. Res. 87 1361
 Google Scholar
Google Scholar
[10] Stevens M H 2001 J. Geophys. Res. 106 3685
 Google Scholar
Google Scholar
[11] Vuitton V, Yelle R V, Anicich V G 2006 Astrophys. J. Lett. 647 L175
 Google Scholar
Google Scholar
[12] Liang M C, Heays A N, Lewis B R, Gibson S T, Yung Y L 2007 Astrophys. J. Lett. 664 L115
 Google Scholar
Google Scholar
[13] Knauth D C, Andersson B G, McCandliss S R, Moos H W 2004 Nature 429 636
 Google Scholar
Google Scholar
[14] Stark G, Huber K P, Yoshino K, Smith P L, Ito K 2005 J. Chem. Phys. 123 214303
 Google Scholar
Google Scholar
[15] Rothman L S, Jacquemart D, Barbe A, et al. 2005 J. Quant. Spectrosc. Radiat. Transfer 96 139
 Google Scholar
Google Scholar
[16] Rothman L S, Gordon I E, Barbe A, et al. 2009 J. Quant. Spectrosc. Radiat. Transfer 110 533
 Google Scholar
Google Scholar
[17] Gordon I E, Rothman L S, Hill C, et al. 2017 J. Quant. Spectrosc. Radiat. Transfer 203 3
 Google Scholar
Google Scholar
[18] Rothman L S, Wattson R B, Gamache R, Schroeder J W, McCann A 1995 Proc. Soc. 2471 105
 Google Scholar
Google Scholar
[19] Rothman L S, Gordon I E, Barber R J, Dothe H, Gamache R R, Goldman A, Perevalov V I, Tashkun S A and Tennyson J 2010 J. Quant. Spectrosc. Radiat. Transfer 111 2139
 Google Scholar
Google Scholar
[20] Lofthus A, Krupenie P H 1977 J. Phys. Chem. Ref. Data 6 113
 Google Scholar
Google Scholar
[21] Stark G, Smith P L, Huber K P, Yoshino K, Ito K 1992 J. Chem. Phys. 97 4809
 Google Scholar
Google Scholar
[22] Haverd V E, Lewis B R, Gibson S T, Stark G 2005 J. Chem. Phys. 123 214304
 Google Scholar
Google Scholar
[23] Robert Wu C Y, Judge D L, Matsui T 2006 J. Geophys. Res. 111 A5
 Google Scholar
Google Scholar
[24] Niu M L, Heays A N, Jones S, Salumbides E J, van Dishoeck E F, De Oliveira N, Nahon L, Ubachs W 2015 J. Mol. Spectrosc. 315 137
 Google Scholar
Google Scholar
[25] Heays A N, Lewis B R, De Oliveira N, Ubachs W 2019 J. Chem. Phys. 151 224305
 Google Scholar
Google Scholar
[26] Spelsberg D, Meyer W 2001 J. Chem. Phys. 115 6438
 Google Scholar
Google Scholar
[27] San-Fabián E, Pastor-Abia L 2003 Theor. Chem. Acc. 110 276
 Google Scholar
Google Scholar
[28] Hochlaf M, Ndome H, Hammoutène D, Vervloet M 2010 J. Phys. B: At. Mol. Opt. Phys. 43 245101
 Google Scholar
Google Scholar
[29] Shi D H, Xing W, Sun J F, Zhu Z L, Liu Y F 2012 Int. J. Quantum Chem. 112 1323
 Google Scholar
Google Scholar
[30] Xin Y, Ding H B 2014 Plasma Sci. Technol. 16 104
 Google Scholar
Google Scholar
[31] Lavín C, Velasco A M, Martín I 2010 Chem. Phys. Lett. 487 38
 Google Scholar
Google Scholar
[32] Lavín C, Velasco A M 2011 Astrophys. J. 739 16
 Google Scholar
Google Scholar
[33] Lavín C, Velasco A M 2016 Astrophys. J. 816 58
 Google Scholar
Google Scholar
[34] Lavín C, Velasco A M 2017 Astrophys. J. Suppl. Ser. 229 19
 Google Scholar
Google Scholar
[35] Velasco A M, Lavín C 2020 Astrophys. J. 899 57
 Google Scholar
Google Scholar
[36] Velasco A M, Alonso J L, Redondo P, Lavín C 2021 Astrophys. J. 922 100
 Google Scholar
Google Scholar
[37] Qin Z, Zhao J, Liu L 2019 Mol. Phys. 117 1
 Google Scholar
Google Scholar
[38] Liang R H, Liu Y M, Li F Y 2021 Phys. Scr. 96 125402
 Google Scholar
Google Scholar
[39] Weck P F, Schweitzer A, Kirby K, Hauschildt P H, Stancil P C 2004 Astrophys. J. 613 567
 Google Scholar
Google Scholar
[40] Werner H J, Knowles P J, Knizia G, et al. 2010 MOLPRO: a Package of ab initio Programs
[41] Le Roy R J 2002 LEVEL 7.5: a Computer Program for Solving the Radial Schrodinger Equation for Bound and Quasibound Levels (University of Waterloo, Chemical Physics Research Report CP-655)
[42] Langhoff S R, Davidson E R 1974 Int. J. Quantum. Chem. 8 61
 Google Scholar
Google Scholar
[43] Werner H J, Knowles P J 1985 J. Chem. Phys. 82 5053
 Google Scholar
Google Scholar
[44] Woon D E, Dunning T H 1995 J. Chem. Phys. 103 4572
 Google Scholar
Google Scholar
[45] Werner H J, Knowles P J 1988 J. Chem. Phys. 89 5803
 Google Scholar
Google Scholar
[46] Knowles P J, Werner H J 1988 Chem. Phys. Lett. 145 514
 Google Scholar
Google Scholar
[47] Moore C E 1975 Natl. Stand. Ref. Data Ser. (U.S. Natl. Bur. Stand) doc. 3 Sect. 5
[48] Müller T, Dallos M, Lischka H, Dubrovay Z, Szalay P G 2001 Theor. Chem. Acc. 105 227
 Google Scholar
Google Scholar
[49] Falzon C T, Chong D P, Wang F 2006 J. Comput. Chem. 27 163
 Google Scholar
Google Scholar
[50] Li X Z, Paldus J 2008 J. Chem. Phys. 129 54104
 Google Scholar
Google Scholar
[51] Huber H P, Herzberg G 1979 Molecular Spectra and Molecular Structure IV Constants of Diatomic Molecules (New York: Van Nostrand) p416
[52] Li X Z, Paldus J 2000 J. Chem. Phys. 113 9966
 Google Scholar
Google Scholar
[53] Li H, Le Roy R J 2007 J. Chem. Phys. 126 224301
 Google Scholar
Google Scholar
[54] Le Roy R J, Huang Y, Jary C 2006 J. Chem. Phys. 125 164310
 Google Scholar
Google Scholar
[55] Edwards S, Roncin J Y, Launay F, Rostas F 1993 J. Mol. Spectrosc. 162 257
 Google Scholar
Google Scholar
计量
- 文章访问数: 5825
- PDF下载量: 77
- 被引次数: 0




















 下载:
下载: