-
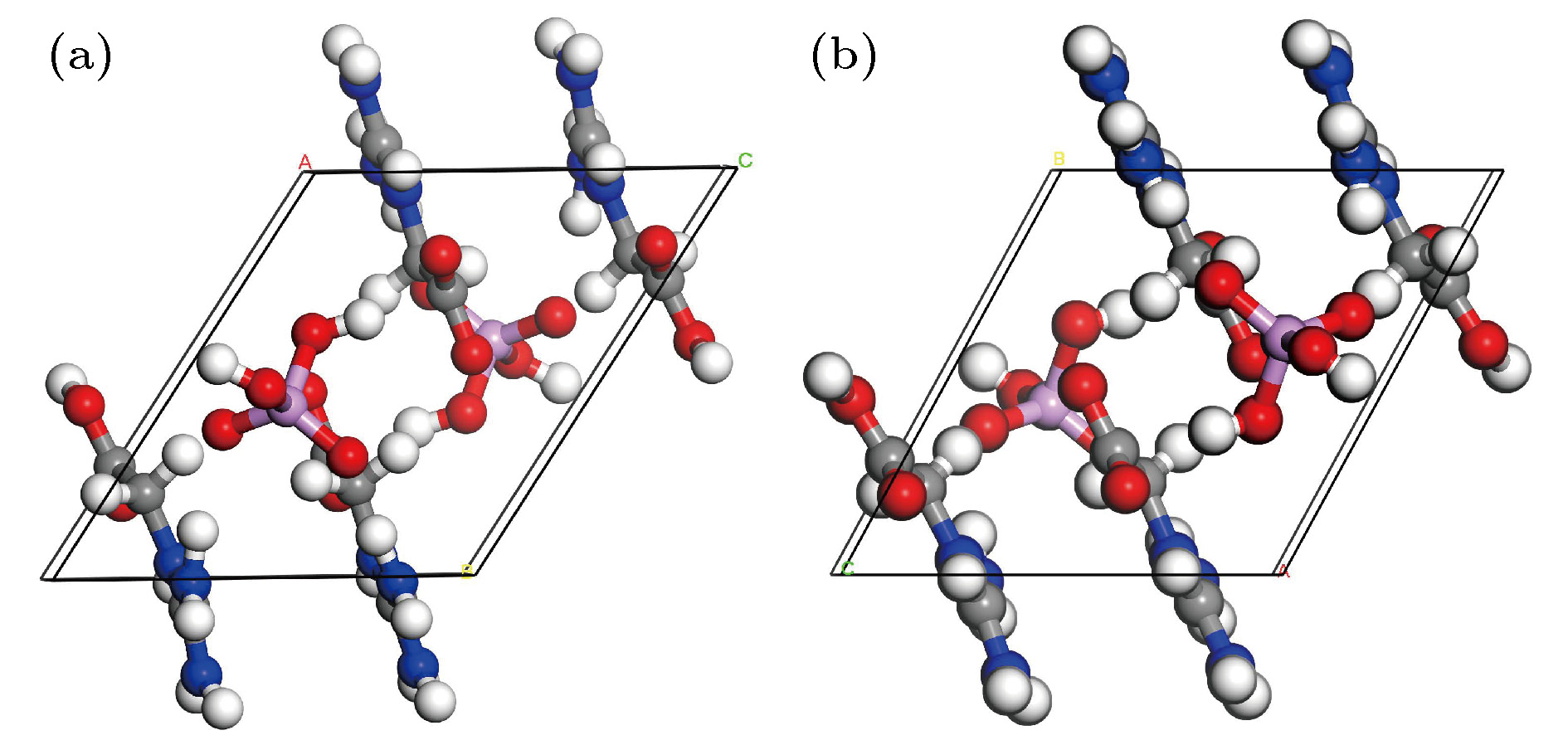
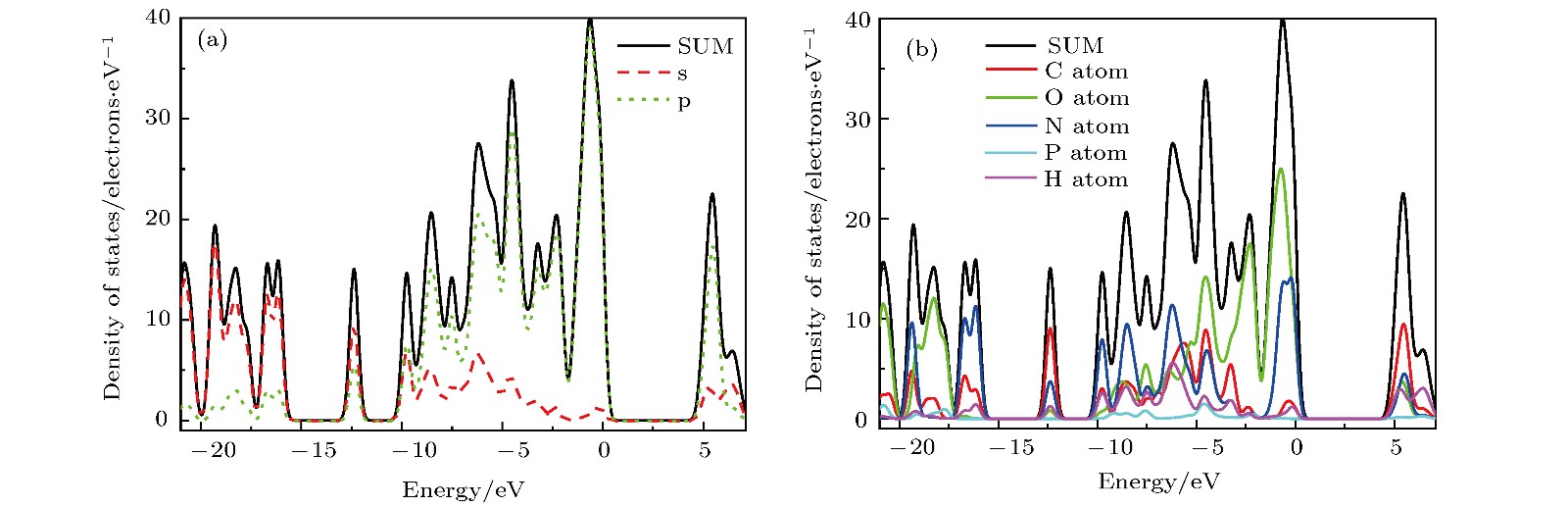
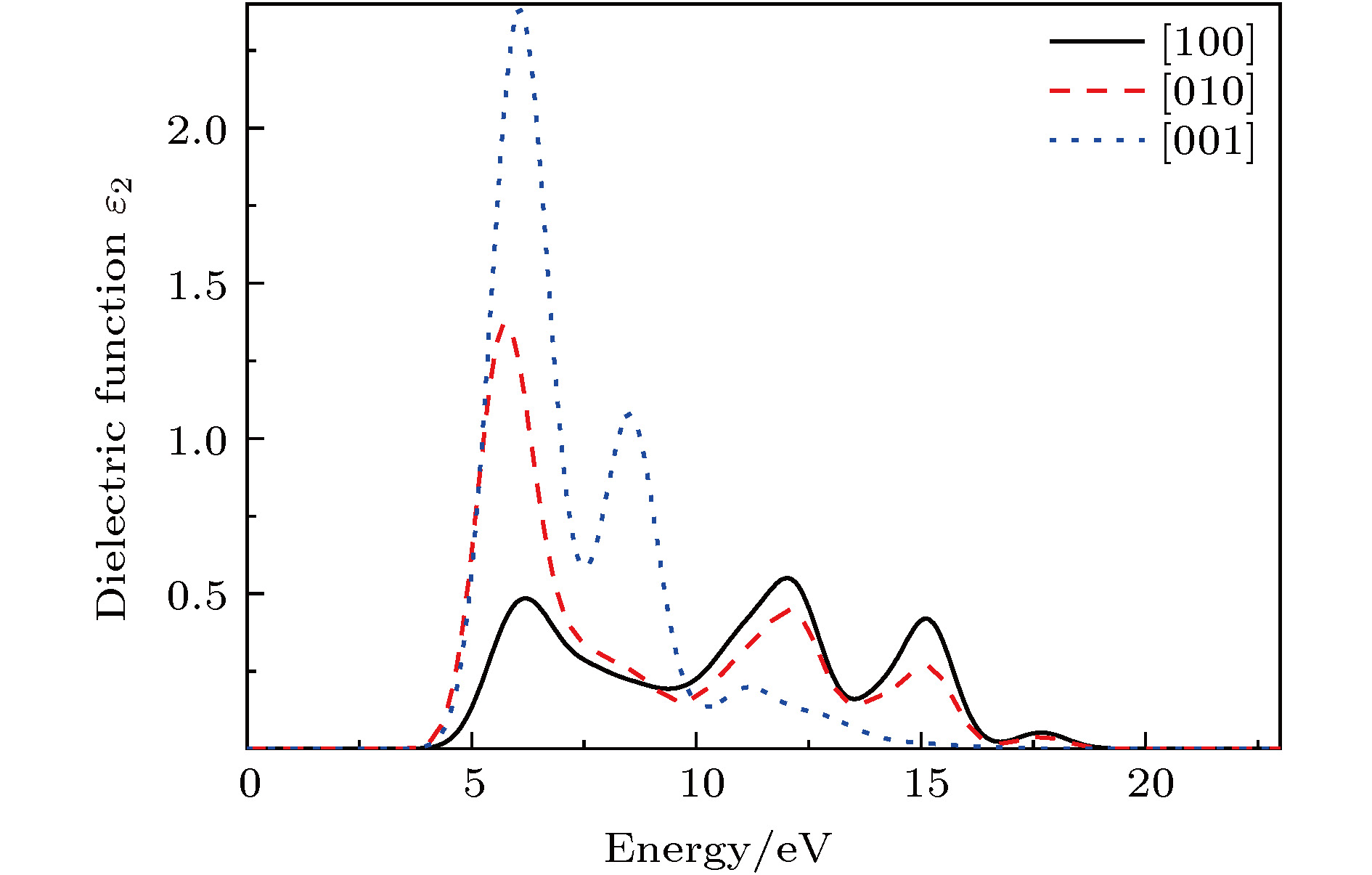
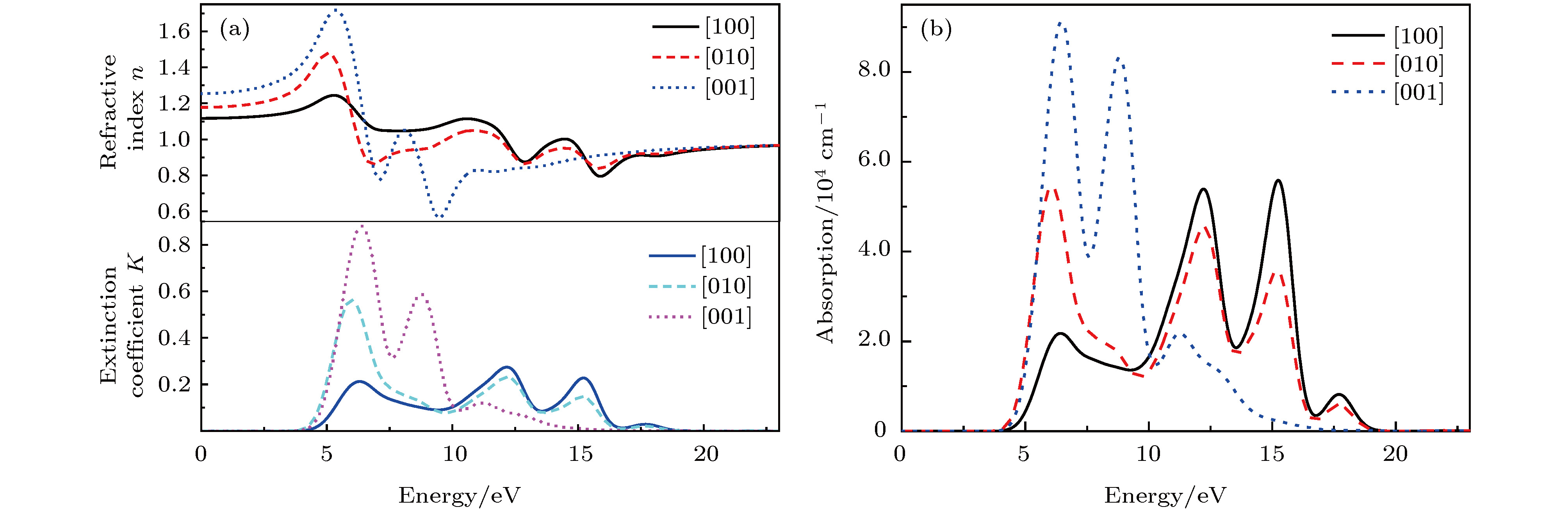
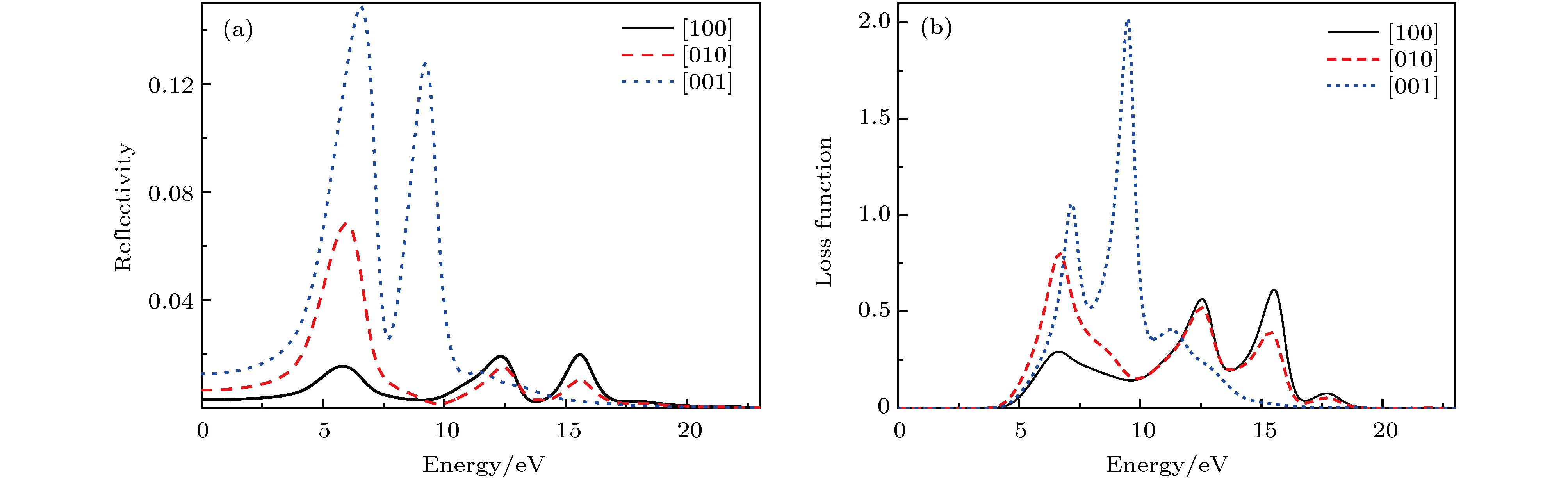
基于磷酸胍基间作用在L-精氨酸磷酸盐晶体特异性与生物化学功能中的重要角色, 已制备了含有磷酸胍基的新晶体磷酸双乙酸胍. 本文采用第一性原理计算了磷酸双乙酸胍晶体的电子结构与三个晶向上的光学性质, 探讨了其中基团间作用与光学性质的关联. 结果表明, 磷酸双乙酸胍晶体能隙为4.77 eV, 远小于磷酸二氢钾晶体, 更易吸收光子, 在胍基、羧基与磷酸根上发生电子跃迁. 磷酸双乙酸胍晶体在[100]和[010]方向光学性质相近, [001]晶向上胍基N-2p在价带内电子跃迁产生强吸收, 能量损失高且分布较窄, 光学应用受到限制, 该研究对理解和研究磷酸双乙酸胍晶体中基团间作用及其光学性质奠定了良好的基础.L-arginine phosphate monohydrate (LAP) crystal is an excellent nonlinear optical material, its effective nonlinear optical coefficient is about 2−3.5 times that of potassium dideuterium phosphate (KDP) crystal, and its conversion efficiency can achieve up to 90%. The deuterated crystal of LAP has a very high laser damage threshold. Thus, once it was considered as a preferred material to replace KDP crystal for laser inertial confinement fusion and other fields. In addition, the LAP crystal has a much higher stimulated Brillouin scattering (SBS) reflectivity than quartz crystal and also has a lower SBS threshold. Moreover, it exhibits a special reversible phase-change in the variable temperature process, and shows an ultra-long spin-lattice relaxation time at solid-state NMR. In a word, the LAP crystal has shown its uniqueness under the action of energy such as light, heat and magnetic field. However, for these special phenomena, there is no reasonable explanation. Phosphate arginine is responsible for the biological energy storage and transfer in invertebrates as an important phosphorus source, which has a similar chemical composition to that of LAP crystal. The special electrostatic or hydrogen bonding interaction between guanidine and phosphate plays an important role in protein molecule interaction and their biochemical functions. Moreover, the conformational transitions of L-arginine molecule in phosphoric acid solution at different energies have been reported, and the fluorescence emission of L-arginine molecule aggregates can be changed by the interaction between phosphoate and guanidine group. The interaction between phosphoate and guanidine group in crystal structure is also studied as a model of biomolecular interaction. In order to further study the mechanism of interaction between phosphoate and guanidine group and the crystal macroscopic properties, phosphate bis-guanidinoacetate (PBGA) crystal containing the similar phosphoate and guanidine groups has been synthesized and reported. In this paper, the geometry parameters, band structure, electronic density of states, and optical properties of PBGA crystal are investigated by first-principles based the density functional theory. The energy gap of PBGA crystal is 4.77 eV, much smaller than 5.96 eV of KDP crystal. Therefore, the photon transition becomes easier and the corresponding photon absorption is relatively large in PBGA crystal. The top states of crystal valence band are mainly composed of the N-2p of guanidine and the O-2p of carboxyl and phosphate groups. There exists the electron interaction among guanidine, carboxyl and phosphate groups. The optical properties of PBGA crystal are similar in the [100] and [010] orientation, whose linear optical properties are better than those of [001] when the incident photon energy is less than 10 eV. The strong energy loss peak at 9.46 eV in the [001] orientation is due to the electronic transition of N-2p on guanidine group in the valence band, and its distribution is narrow. Thus the optical properties of [001] orientation are limited. The present research establishes a good foundation for further understanding and studying the intergroup interactions and optical properties in PBGA crystal.
-
Keywords:
- phosphate bis-guanidinoacetate /
- first principles /
- electronic structure /
- optical properties
[1] 王镜岩, 朱圣庚, 徐长法 2002 生物化学 (第三版下册) (北京: 高等教育出版社) 第41页
Wang J Y, Zhu S G, Xu C F 2002 Biochemistry (3rd Ed.) Vol. 2 (Beijing: Higher Education Press) p41 (in Chinese)
[2] Bailey D M, Peck L S, Bock C, Portner H 2003 Physiol. Biochem. Zool. 76 622
 Google Scholar
Google Scholar
[3] Senior A E, Nadanaciva S, Weber J 2002 Biochem. Biophys. Acta 1553 188
 Google Scholar
Google Scholar
[4] Xian L, Liu S, Ma Y, Lu G 2007 Spectrochim. Acta Part A 67 368
 Google Scholar
Google Scholar
[5] Mandell D J, Chorny I, Groban E S, Wong S E, Levine E, Rapp C S, Jacobson M P 2007 J. Am. Chem. Soc. 129 820
 Google Scholar
Google Scholar
[6] Tang M, Waring A J, Lehrer R I, Hong M 2008 Angew. Chem. Int. Ed. 47 3202
 Google Scholar
Google Scholar
[7] Cotton F A, Day V W, Hazen E E, Larsen S 1973 J. Am. Chem. Soc. 95 4834
 Google Scholar
Google Scholar
[8] 许东, 蒋民华, 谭忠恪 1983 化学学报 41 570
Xu D, Jiang M H, Tan Z K 1983 Acta Chim. Sin. 41 570
[9] Eimerl D, Velsko S, Davis L, Wang F, Loiacono G, Kennedy G 1989 IEEE J. Quantum Electron. 25 179
 Google Scholar
Google Scholar
[10] Eimerl D 1985 LLNL Report UCID 20565 92
[11] Yoshimura M, Mori Y, Sasaki T, Yoshida H, Nakatsuka M 1998 J. Opt. Soc. Am. 15 446
 Google Scholar
Google Scholar
[12] 孙贵花 2011 博士学位论文 (济南: 山东大学)
Sun G H 2011 Ph. D. Dissertation (Jinan: Shandong University) (in Chinese)
[13] Wang L N, Zhang G H, Wang X Q, Wang L, Liu X T, Jin L T, Xu D 2012 J. Mol. Strct. 1026 71
 Google Scholar
Google Scholar
[14] Liu X T, Wang L, Wang L N, Zhang G H, Wang X Q, Xu D 2014 Int. J. Mater. Sci. 4 39
 Google Scholar
Google Scholar
[15] 王磊 2014 博士学位论文 (济南: 山东大学)
Wang L 2014 Ph. D. Dissertation (Jinan: Shandong University) (in Chinese)
[16] Segall M D, Lindan P J D, Probert M J, Pickard C J, Hasnip P J, Clark S J, Payne M C 2002 J. Phys. Condens. Mater. 14 2717
 Google Scholar
Google Scholar
[17] Perdew J P, Wang Y 1992 Phys. Rev. B 45 13244
 Google Scholar
Google Scholar
[18] Vanderbilt D 1990 Phys. Rev. B 41 7892
 Google Scholar
Google Scholar
[19] 段满益, 徐明, 周海平, 陈青云, 胡志刚, 董成军 2008 57 6520
 Google Scholar
Google Scholar
Duan M Y, Xu M, Zhou H P, Chen Q Y, Hu Z G, Dong C J 2008 Acta Phys. Sin. 57 6520
 Google Scholar
Google Scholar
[20] Monkhorst H J, Pack J D 1976 Phys. Rev. B 13 5188
 Google Scholar
Google Scholar
[21] 沈学础 1992 半导体光学性质 (北京: 科学出版社) 第24页
Shen X C 1992 Optical Property of Semiconductor (Beijing: Science Press) p24 (in Chinese)
[22] 徐大庆, 赵子涵, 李培咸, 王超, 张岩, 刘树林, 童军 2018 67 087501
 Google Scholar
Google Scholar
Xu D Q, Zhao Z H, Li P X, Wang C, Zhang Y, Liu S L, Tong J 2018 Acta Phys. Sin. 67 087501
 Google Scholar
Google Scholar
[23] Zhang Q, Chen F, Kioussis N, Demos S G, Radousky H B 2001 Phys. Rev. B 65 024108
 Google Scholar
Google Scholar
-
-
[1] 王镜岩, 朱圣庚, 徐长法 2002 生物化学 (第三版下册) (北京: 高等教育出版社) 第41页
Wang J Y, Zhu S G, Xu C F 2002 Biochemistry (3rd Ed.) Vol. 2 (Beijing: Higher Education Press) p41 (in Chinese)
[2] Bailey D M, Peck L S, Bock C, Portner H 2003 Physiol. Biochem. Zool. 76 622
 Google Scholar
Google Scholar
[3] Senior A E, Nadanaciva S, Weber J 2002 Biochem. Biophys. Acta 1553 188
 Google Scholar
Google Scholar
[4] Xian L, Liu S, Ma Y, Lu G 2007 Spectrochim. Acta Part A 67 368
 Google Scholar
Google Scholar
[5] Mandell D J, Chorny I, Groban E S, Wong S E, Levine E, Rapp C S, Jacobson M P 2007 J. Am. Chem. Soc. 129 820
 Google Scholar
Google Scholar
[6] Tang M, Waring A J, Lehrer R I, Hong M 2008 Angew. Chem. Int. Ed. 47 3202
 Google Scholar
Google Scholar
[7] Cotton F A, Day V W, Hazen E E, Larsen S 1973 J. Am. Chem. Soc. 95 4834
 Google Scholar
Google Scholar
[8] 许东, 蒋民华, 谭忠恪 1983 化学学报 41 570
Xu D, Jiang M H, Tan Z K 1983 Acta Chim. Sin. 41 570
[9] Eimerl D, Velsko S, Davis L, Wang F, Loiacono G, Kennedy G 1989 IEEE J. Quantum Electron. 25 179
 Google Scholar
Google Scholar
[10] Eimerl D 1985 LLNL Report UCID 20565 92
[11] Yoshimura M, Mori Y, Sasaki T, Yoshida H, Nakatsuka M 1998 J. Opt. Soc. Am. 15 446
 Google Scholar
Google Scholar
[12] 孙贵花 2011 博士学位论文 (济南: 山东大学)
Sun G H 2011 Ph. D. Dissertation (Jinan: Shandong University) (in Chinese)
[13] Wang L N, Zhang G H, Wang X Q, Wang L, Liu X T, Jin L T, Xu D 2012 J. Mol. Strct. 1026 71
 Google Scholar
Google Scholar
[14] Liu X T, Wang L, Wang L N, Zhang G H, Wang X Q, Xu D 2014 Int. J. Mater. Sci. 4 39
 Google Scholar
Google Scholar
[15] 王磊 2014 博士学位论文 (济南: 山东大学)
Wang L 2014 Ph. D. Dissertation (Jinan: Shandong University) (in Chinese)
[16] Segall M D, Lindan P J D, Probert M J, Pickard C J, Hasnip P J, Clark S J, Payne M C 2002 J. Phys. Condens. Mater. 14 2717
 Google Scholar
Google Scholar
[17] Perdew J P, Wang Y 1992 Phys. Rev. B 45 13244
 Google Scholar
Google Scholar
[18] Vanderbilt D 1990 Phys. Rev. B 41 7892
 Google Scholar
Google Scholar
[19] 段满益, 徐明, 周海平, 陈青云, 胡志刚, 董成军 2008 57 6520
 Google Scholar
Google Scholar
Duan M Y, Xu M, Zhou H P, Chen Q Y, Hu Z G, Dong C J 2008 Acta Phys. Sin. 57 6520
 Google Scholar
Google Scholar
[20] Monkhorst H J, Pack J D 1976 Phys. Rev. B 13 5188
 Google Scholar
Google Scholar
[21] 沈学础 1992 半导体光学性质 (北京: 科学出版社) 第24页
Shen X C 1992 Optical Property of Semiconductor (Beijing: Science Press) p24 (in Chinese)
[22] 徐大庆, 赵子涵, 李培咸, 王超, 张岩, 刘树林, 童军 2018 67 087501
 Google Scholar
Google Scholar
Xu D Q, Zhao Z H, Li P X, Wang C, Zhang Y, Liu S L, Tong J 2018 Acta Phys. Sin. 67 087501
 Google Scholar
Google Scholar
[23] Zhang Q, Chen F, Kioussis N, Demos S G, Radousky H B 2001 Phys. Rev. B 65 024108
 Google Scholar
Google Scholar
计量
- 文章访问数: 11387
- PDF下载量: 59
- 被引次数: 0














 下载:
下载: