-
According to the phenomenon that the solubility of CH3NH3PbCl3 decreases with the increase of temperature in different solvents, CH3NH3PbCl3 perovskite single crystal with a maximum dimension of 11 mm × 11 mm × 2 mm is grown by introducing a high-quality seed crystal via the seed-induced inverse temperature crystallization method in this work. X-ray diffraction and Rietveld refinements show that the full widths at half maximum (FWHM) of CH3NH3PbCl3 single crystal diffraction peaks are 0.1527°, 0.1353°, 0.2295° and 0.3452°, corresponding to the crystal plane indices of (100), (200), (300) and (400), respectively. And there are no miscellaneous peaks, indicating a good crystal quality. As a result, CH3NH3PbCl3 single crystal is of cubic phase at room temperature, its space group belongs to Pm
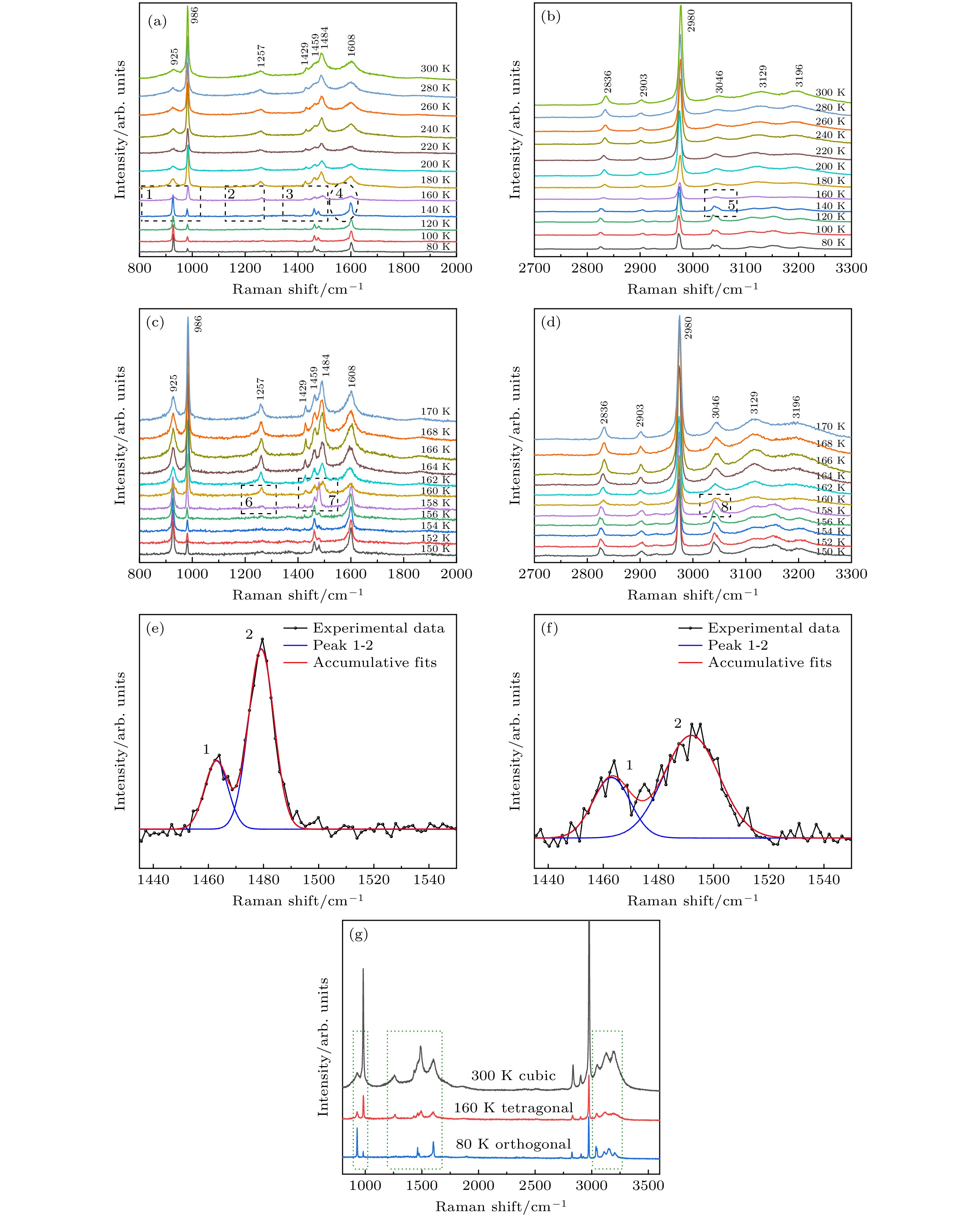
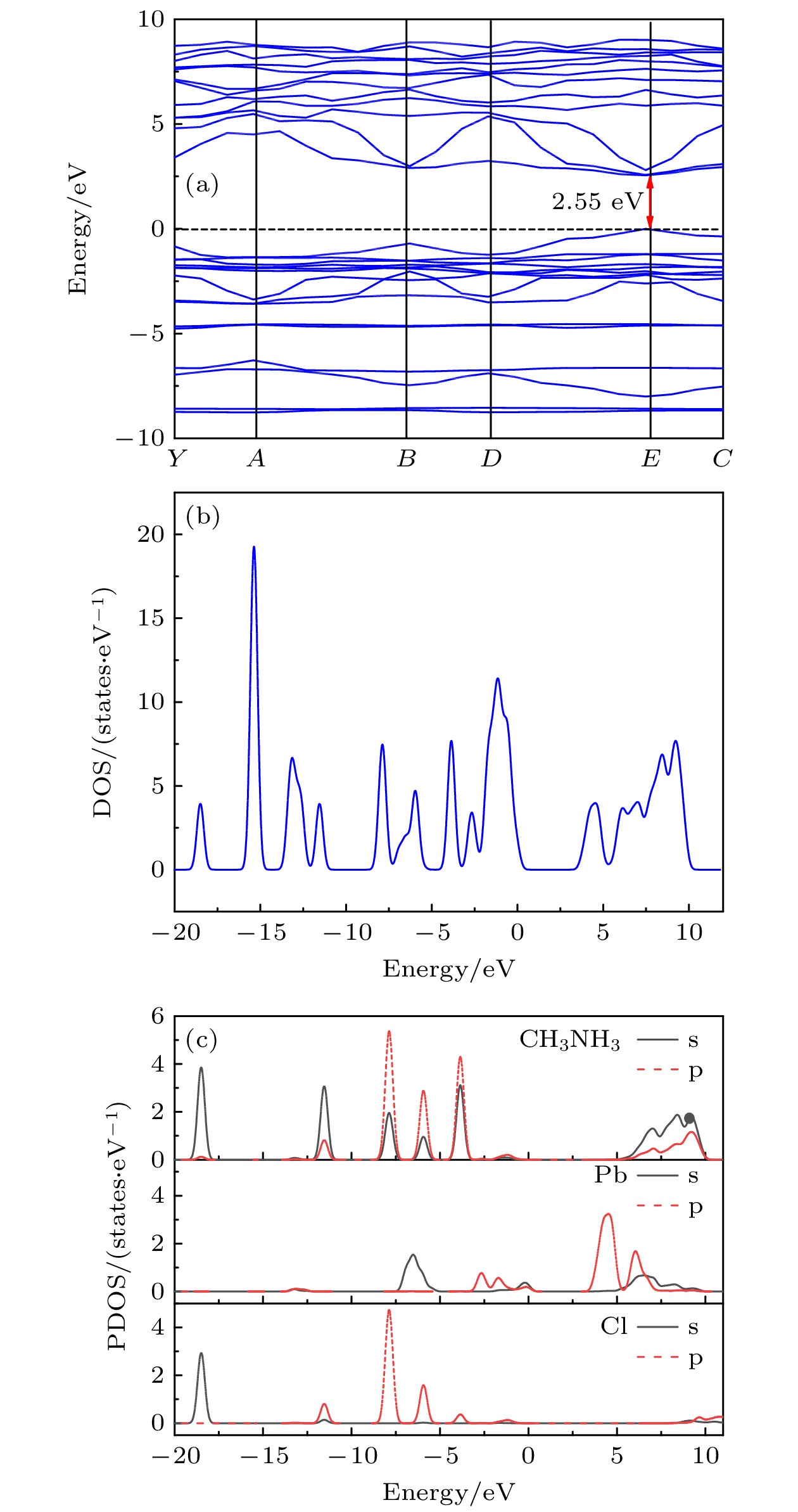
$ \bar{3} $ m, and the lattice constant is a = 0.56877 nm. The surface morphology and growth mechanism of CH3NH3PbCl3 crystal are investigated by using a polarizing microscope. It is found that its growth mechanism follows the step horizontal growing mechanism of smooth interface, and its growth direction (that is, step movement direction) is along the outward normal direction of the step. The structural symmetry of CH3NH3PbCl3 crystal is studied by variable temperature Raman spectroscopy, which reveals an orthogonal-tetragonal phase transition at 160 K. But the tetragonal phase structure is not stable, and its temperature range is very narrow. As temperature rises gradually, the tetragonal phase again transforms into a cubic phase (Pm$\bar{3}$ m). Results of UV-Vis-NIR absorption and photoluminescence spectra show that the absorption cutoff of CH3NH3PbCl3 crystal is about 442 nm, and the photoluminescence peak is 450 nm. Thereupon, its band gap is obtained to be about 2.93 eV by a linear fit of Tauc formula, which is slightly higher than the theoretical value of 2.55 eV calculated by first principles simulation. We believe that it is related to the seed crystal, which is introduced into the crystal growth process as the core of heterogeneous nucleation and thus making the lattice more distorted. The lower the lattice symmetry of CH3NH3PbCl3, the larger the band gap is, that is, the lattice symmetry determines the degree of distortion for inorganic PbCl6 octahedral frameworks, resulting in an increase of band gap for CH3NH3PbCl3.-
Keywords:
- CH3NH3PbCl3 single crystal /
- seed-induced inverse temperature crystallization method /
- step horizontal growing mechanism /
- first-principles calculations
[1] Turedi B, Lintangpradipto M N, Sandberg O J, Yazmaciyan A, Matt G J, Alsalloum A Y, Almasabi K, Sakhatskyi K, Yakunin S, Zheng X, Naphade R, Nematulloev S, Yeddu V, Baran D, Armin A, Saidaminov M I, Kovalenko M V, Mohammed O F, Bakr O M 2022 Adv. Mater. 34 2202390
 Google Scholar
Google Scholar
[2] Xing G, Mathews N, Sun S, Lim S S, Lam Y M, Grätzel M, Mhaisalkar S, Sum T C 2013 Science 342 344
 Google Scholar
Google Scholar
[3] Wang S, Yang F, Zhu J, Cao Q, Zhong Y, Wang A, Du W, Liu X 2020 Sci. China Mater. 63 1438
 Google Scholar
Google Scholar
[4] Stranks S D, Eperon G E, Grancini G, Menelaou C, Alcocer M J P, Leijtens T, Herz L M, Petrozza A, Snaith H J 2013 Science 342 341
 Google Scholar
Google Scholar
[5] Zhang Y, Liu Y, Liu S 2022 Adv. Funct. Mater. 32 2210335
 Google Scholar
Google Scholar
[6] Chen H, Ye F, Tang W, He J, Yin M, Wang Y, Xie F, Bi E, Yang X, Grätzel M, Han L 2017 Nature 550 92
 Google Scholar
Google Scholar
[7] Li M, Zhao C, Wang Z, Zhang C, Lee H K H, Pockett A, Barbé J, Tsoi W C, Yang Y, Carnie M J, Gao X, Yang W, Durrant J R, Liao L, Jain S M 2018 Adv. Energy Mater. 8 1801509
 Google Scholar
Google Scholar
[8] Wang Y, Chen W, Wang L, Tu B, Chen T, Liu B, Yang K, Koh C W, Zhang X, Sun H, Chen G, Feng X, Woo H Y, Djurišic´ A B, He Z, Guo X 2019 Adv. Mater. 31 1902781
 Google Scholar
Google Scholar
[9] Quan L N, García de Arquer F P, Sabatini R P, Sargent E H 2018 Adv. Mater. 30 1801996
 Google Scholar
Google Scholar
[10] Cao Y, Wang N, Tian H, Guo J, Wei Y, Chen H, Miao Y, Zou W, Pan K, He Y, Cao H, Ke Y, Xu M, Wang Y, Yang M, Du K, Fu Z, Kong D, Dai D, Jin Y, Li G, Li H, Peng Q, Wang J, Huang W 2018 Nature 562 249
 Google Scholar
Google Scholar
[11] Zhang Q, Su R, Liu X, Xing J, Sum T C, Xiong Q 2016 Adv. Funct. Mater. 26 6238
 Google Scholar
Google Scholar
[12] Wang K, Wang S, Xiao S, Song Q 2018 Adv. Optical Mater. 6 1800278
 Google Scholar
Google Scholar
[13] Li D, Cheng H, Wang Y, Zhao Z, Wang G, Wu H, He Q, Huang Y, Duan X 2017 Adv. Mater. 29 1601959
 Google Scholar
Google Scholar
[14] Yu W, Li F, Yu L, Niazi M R, Zou Y, Corzo D, Basu A, Ma C, Dey S, Tietze M L, Buttner U, Wang X, Wang Z, Hedhili M N, Guo C, Wu T, Amassian A 2018 Nat. Commun. 9 5354
 Google Scholar
Google Scholar
[15] Tian W, Zhou H, Li L 2017 Small 13 1702107
 Google Scholar
Google Scholar
[16] Pan W, Wei H, Yang B 2020 Front. Chem. 8 268
 Google Scholar
Google Scholar
[17] Andričević P, Frajtag P, Lamirand V P, Pautz A, Kollár M, Náfrádi B, Sienkiewicz A, Garma T, Forró L, Horváth E 2021 Adv. Sci. 8 2001882
 Google Scholar
Google Scholar
[18] Ma L, Yan Z, Zhou X, Pi Y, Du Y, Huang J, Wang K, Wu K, Zhuang C, Han X 2021 Nat. Commun. 12 2023
 Google Scholar
Google Scholar
[19] Shen H, Nan R, Jian Z, Li X 2019 J. Mater. Sci. 54 11596
 Google Scholar
Google Scholar
[20] Wang W, Meng H, Qi H, Xu H, Du W, Yang Y, Yi Y, Jing S, Xu S, Hong F, Qin J, Huang J, Xu Z, Zhu Y, Xu R, Lai J, Xu F, Wang L, Zhu J 2020 Adv. Mater. 32 2001540
 Google Scholar
Google Scholar
[21] Ryu S, Noh J H, Jeon N J, Kim Y C, Yang W S, Seoa J, Seok S I 2014 Energy Environ. Sci. 7 2614
 Google Scholar
Google Scholar
[22] Cheng X, Jing L, Zhao Y, Du S, Ding J, Zhou T 2018 J. Mater. Chem. C 6 1579
 Google Scholar
Google Scholar
[23] Zheng E, Yuh B, Tosado G A, Yu Q 2017 J. Mater. Chem. C 5 3796
 Google Scholar
Google Scholar
[24] Liu Y, Yang Z, Cui D, Ren X, Sun J, Liu X, Zhang J, Wei Q, Fan H, Yu F, Zhang X, Zhao C, Liu S 2015 Adv. Mater. 27 5176
 Google Scholar
Google Scholar
[25] Ding J, Cheng X, Jing L, Zhou T, Zhao Y, Du S 2018 ACS Appl. Mater. Interfaces 10 845
 Google Scholar
Google Scholar
[26] Mosconi E, Amat A, Nazeeruddin M K, Grätzel M, Angelis F D 2013 J. Phys. Chem. C 117 13902
 Google Scholar
Google Scholar
[27] Zhang F, Zhong H, Chen C, Wu X, Hu X, Huang H, Han J, Zou B, Dong Y 2015 ACS Nano 9 4533
 Google Scholar
Google Scholar
[28] Bernasconi A, Page K, Dai Z, Tan L Z, Rappe A M, Malavasi L 2018 J. Phys. Chem. C 122 28265
 Google Scholar
Google Scholar
[29] Songvilay M, Wang Z, Sakai V G, Guidi T, Bari M, Ye Z G, Xu G, Brown K L, Gehring P M, Stock C 2019 Phys. Rev. Mater. 3 125406
 Google Scholar
Google Scholar
[30] Ivanovska T, Quarti C, Grancini G, Petrozza A, Angelis F D, Milani A, Ruani G 2016 Chem. Sus. Chem 9 2994
 Google Scholar
Google Scholar
[31] Wang H, Nan R, Jian Z, Jin C, Wei Y, Bai Y, Li H 2021 Mater. Sci. Semicond. Process. 135 106107
 Google Scholar
Google Scholar
[32] Singleton J, Rabe K M 2002 Phys. Today 55 61
 Google Scholar
Google Scholar
-
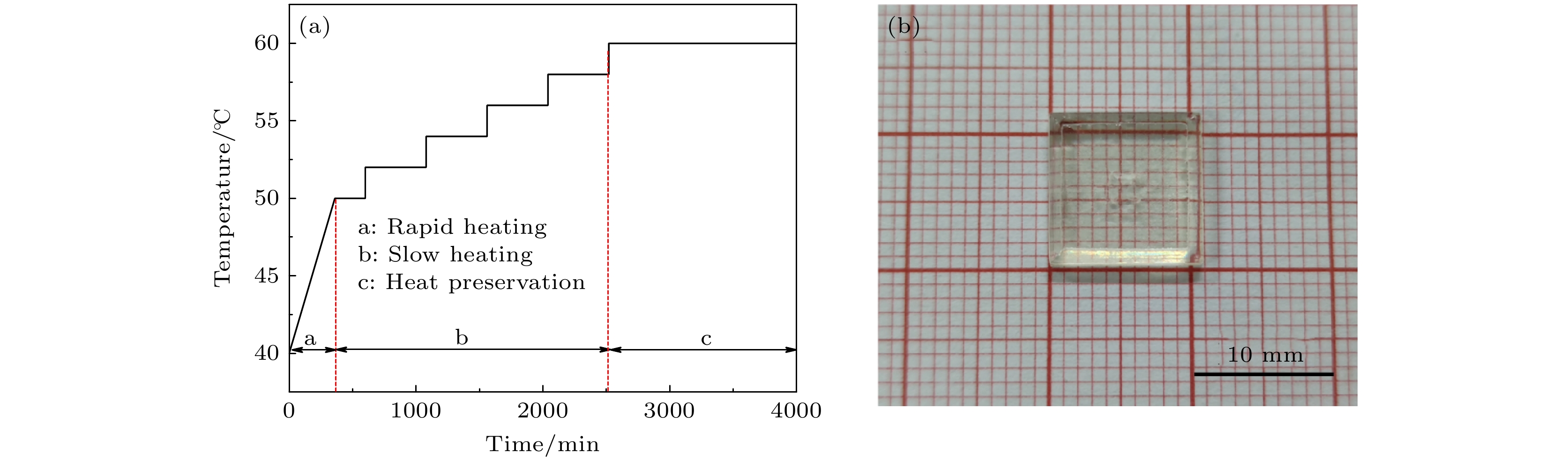
图 1 籽晶诱导逆温差结晶法生长CH3NH3PbCl3单晶 (a)原理示意图; (b) CH3NH3PbCl3在DMF和DMSO混合溶剂中的溶解度曲线
Figure 1. CH3NH3PbCl3 single crystal grown by seed-induced inverse temperature crystallization method: (a) Schematic diagram of principle; (b) temperature-dependent solubility curve of CH3NH3PbCl3 in DMF+DMSO mixed solution.
图 3 籽晶诱导逆温差结晶法生长CH3NH3PbCl3单晶 (a) 单晶XRD图谱; (b)粉末XRD与Material studio模拟的标准XRD图谱; (c) 粉末XRD Rietveld精修结果(Rp = 12.6%, Rwp = 18.5%)
Figure 3. CH3NH3PbCl3 single crystal grown by seed-induced inverse temperature crystallization method: (a) XRD pattern of single crystal; (b) powder XRD and standard XRD pattern simulated by Material studio; (c) Rietveld result of powder XRD pattern (Rp = 12.6%, Rwp = 18.5%).
图 5 (a) CH3NH3PbCl3单晶的UV-Vis-NIR吸收光谱和PL光谱; (b) 通过拟合得到的帯隙值(蓝色散点是实验数据, 黑色实线是拟合结果Eg = 2.93 eV)
Figure 5. (a) UV-Vis-NIR absorption and PL spectra of CH3NH3PbCl3 single crystal; (b) band gap obtained by fitting (Blue scatter dots are the experimental data, and the black solid line is the fitting result of Eg = 2.93 eV).
图 6 CH3NH3PbCl3单晶的变温拉曼光谱 (a), (b) 80—300 K; (c), (d) 150—170 K; (e) 158 K; (f) 160 K; (g) 不同温度下CH3NH3PbCl3晶体结构(立方/四方/正交)的拉曼谱
Figure 6. Temperature-dependent Raman spectra of CH3NH3PbCl3 single crystal: (a), (b) 80–300 K; (c), (d) 150–170 K; (e) 158 K; (f) 160 K; (g) Raman spectra of CH3NH3PbCl3 crystal structures (cubic/tetragonal/orthogonal) at different temperatures.
-
[1] Turedi B, Lintangpradipto M N, Sandberg O J, Yazmaciyan A, Matt G J, Alsalloum A Y, Almasabi K, Sakhatskyi K, Yakunin S, Zheng X, Naphade R, Nematulloev S, Yeddu V, Baran D, Armin A, Saidaminov M I, Kovalenko M V, Mohammed O F, Bakr O M 2022 Adv. Mater. 34 2202390
 Google Scholar
Google Scholar
[2] Xing G, Mathews N, Sun S, Lim S S, Lam Y M, Grätzel M, Mhaisalkar S, Sum T C 2013 Science 342 344
 Google Scholar
Google Scholar
[3] Wang S, Yang F, Zhu J, Cao Q, Zhong Y, Wang A, Du W, Liu X 2020 Sci. China Mater. 63 1438
 Google Scholar
Google Scholar
[4] Stranks S D, Eperon G E, Grancini G, Menelaou C, Alcocer M J P, Leijtens T, Herz L M, Petrozza A, Snaith H J 2013 Science 342 341
 Google Scholar
Google Scholar
[5] Zhang Y, Liu Y, Liu S 2022 Adv. Funct. Mater. 32 2210335
 Google Scholar
Google Scholar
[6] Chen H, Ye F, Tang W, He J, Yin M, Wang Y, Xie F, Bi E, Yang X, Grätzel M, Han L 2017 Nature 550 92
 Google Scholar
Google Scholar
[7] Li M, Zhao C, Wang Z, Zhang C, Lee H K H, Pockett A, Barbé J, Tsoi W C, Yang Y, Carnie M J, Gao X, Yang W, Durrant J R, Liao L, Jain S M 2018 Adv. Energy Mater. 8 1801509
 Google Scholar
Google Scholar
[8] Wang Y, Chen W, Wang L, Tu B, Chen T, Liu B, Yang K, Koh C W, Zhang X, Sun H, Chen G, Feng X, Woo H Y, Djurišic´ A B, He Z, Guo X 2019 Adv. Mater. 31 1902781
 Google Scholar
Google Scholar
[9] Quan L N, García de Arquer F P, Sabatini R P, Sargent E H 2018 Adv. Mater. 30 1801996
 Google Scholar
Google Scholar
[10] Cao Y, Wang N, Tian H, Guo J, Wei Y, Chen H, Miao Y, Zou W, Pan K, He Y, Cao H, Ke Y, Xu M, Wang Y, Yang M, Du K, Fu Z, Kong D, Dai D, Jin Y, Li G, Li H, Peng Q, Wang J, Huang W 2018 Nature 562 249
 Google Scholar
Google Scholar
[11] Zhang Q, Su R, Liu X, Xing J, Sum T C, Xiong Q 2016 Adv. Funct. Mater. 26 6238
 Google Scholar
Google Scholar
[12] Wang K, Wang S, Xiao S, Song Q 2018 Adv. Optical Mater. 6 1800278
 Google Scholar
Google Scholar
[13] Li D, Cheng H, Wang Y, Zhao Z, Wang G, Wu H, He Q, Huang Y, Duan X 2017 Adv. Mater. 29 1601959
 Google Scholar
Google Scholar
[14] Yu W, Li F, Yu L, Niazi M R, Zou Y, Corzo D, Basu A, Ma C, Dey S, Tietze M L, Buttner U, Wang X, Wang Z, Hedhili M N, Guo C, Wu T, Amassian A 2018 Nat. Commun. 9 5354
 Google Scholar
Google Scholar
[15] Tian W, Zhou H, Li L 2017 Small 13 1702107
 Google Scholar
Google Scholar
[16] Pan W, Wei H, Yang B 2020 Front. Chem. 8 268
 Google Scholar
Google Scholar
[17] Andričević P, Frajtag P, Lamirand V P, Pautz A, Kollár M, Náfrádi B, Sienkiewicz A, Garma T, Forró L, Horváth E 2021 Adv. Sci. 8 2001882
 Google Scholar
Google Scholar
[18] Ma L, Yan Z, Zhou X, Pi Y, Du Y, Huang J, Wang K, Wu K, Zhuang C, Han X 2021 Nat. Commun. 12 2023
 Google Scholar
Google Scholar
[19] Shen H, Nan R, Jian Z, Li X 2019 J. Mater. Sci. 54 11596
 Google Scholar
Google Scholar
[20] Wang W, Meng H, Qi H, Xu H, Du W, Yang Y, Yi Y, Jing S, Xu S, Hong F, Qin J, Huang J, Xu Z, Zhu Y, Xu R, Lai J, Xu F, Wang L, Zhu J 2020 Adv. Mater. 32 2001540
 Google Scholar
Google Scholar
[21] Ryu S, Noh J H, Jeon N J, Kim Y C, Yang W S, Seoa J, Seok S I 2014 Energy Environ. Sci. 7 2614
 Google Scholar
Google Scholar
[22] Cheng X, Jing L, Zhao Y, Du S, Ding J, Zhou T 2018 J. Mater. Chem. C 6 1579
 Google Scholar
Google Scholar
[23] Zheng E, Yuh B, Tosado G A, Yu Q 2017 J. Mater. Chem. C 5 3796
 Google Scholar
Google Scholar
[24] Liu Y, Yang Z, Cui D, Ren X, Sun J, Liu X, Zhang J, Wei Q, Fan H, Yu F, Zhang X, Zhao C, Liu S 2015 Adv. Mater. 27 5176
 Google Scholar
Google Scholar
[25] Ding J, Cheng X, Jing L, Zhou T, Zhao Y, Du S 2018 ACS Appl. Mater. Interfaces 10 845
 Google Scholar
Google Scholar
[26] Mosconi E, Amat A, Nazeeruddin M K, Grätzel M, Angelis F D 2013 J. Phys. Chem. C 117 13902
 Google Scholar
Google Scholar
[27] Zhang F, Zhong H, Chen C, Wu X, Hu X, Huang H, Han J, Zou B, Dong Y 2015 ACS Nano 9 4533
 Google Scholar
Google Scholar
[28] Bernasconi A, Page K, Dai Z, Tan L Z, Rappe A M, Malavasi L 2018 J. Phys. Chem. C 122 28265
 Google Scholar
Google Scholar
[29] Songvilay M, Wang Z, Sakai V G, Guidi T, Bari M, Ye Z G, Xu G, Brown K L, Gehring P M, Stock C 2019 Phys. Rev. Mater. 3 125406
 Google Scholar
Google Scholar
[30] Ivanovska T, Quarti C, Grancini G, Petrozza A, Angelis F D, Milani A, Ruani G 2016 Chem. Sus. Chem 9 2994
 Google Scholar
Google Scholar
[31] Wang H, Nan R, Jian Z, Jin C, Wei Y, Bai Y, Li H 2021 Mater. Sci. Semicond. Process. 135 106107
 Google Scholar
Google Scholar
[32] Singleton J, Rabe K M 2002 Phys. Today 55 61
 Google Scholar
Google Scholar
Catalog
Metrics
- Abstract views: 6417
- PDF Downloads: 294
- Cited By: 0

















 DownLoad:
DownLoad: