-
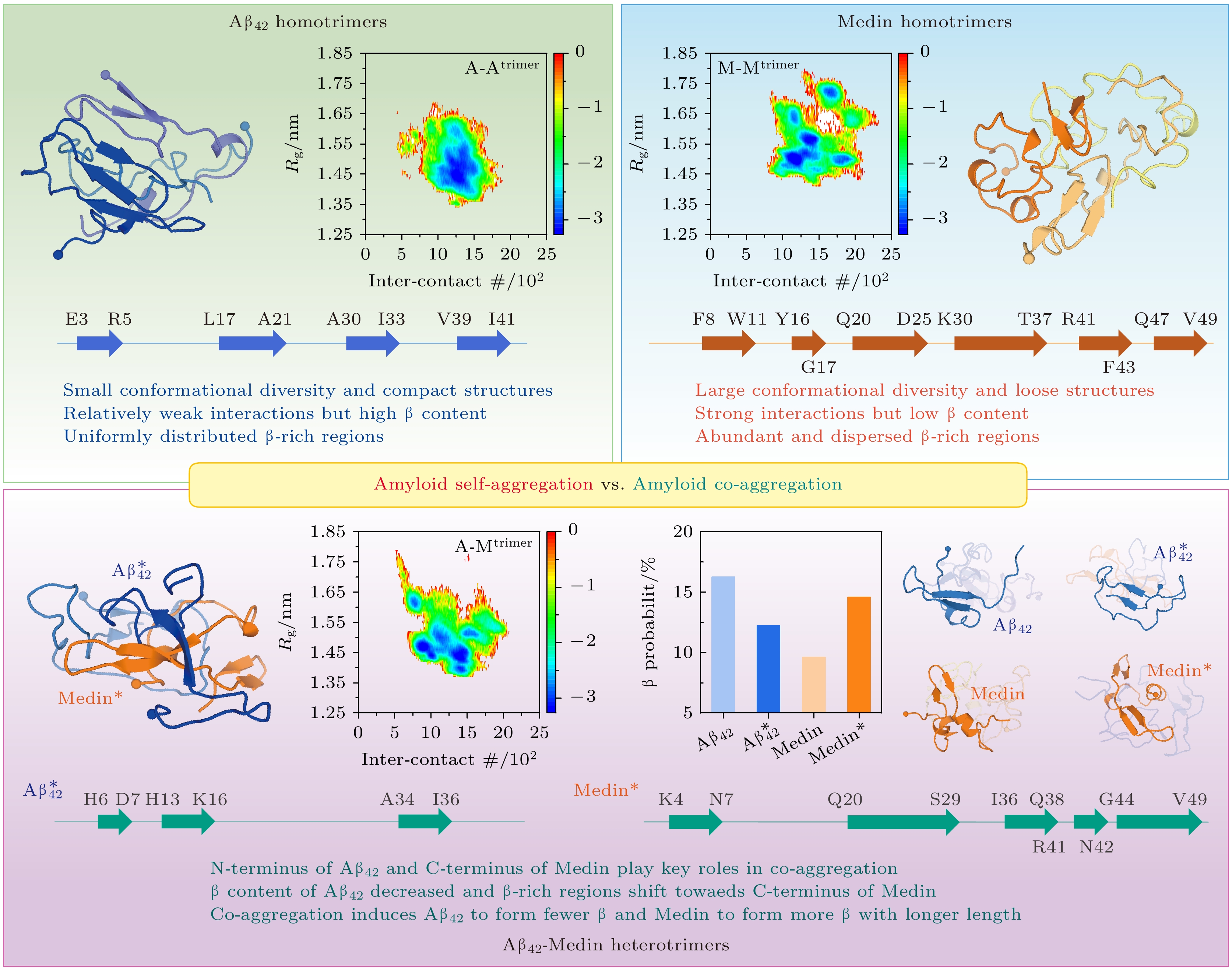
Medin淀粉样蛋白的聚集导致动脉壁退化和脑血管功能障碍, 参与多种血管疾病的发生与发展. 在血管性痴呆或阿尔茨海默病患者的脑小动脉中发现Medin聚集体增加, 且Medin与血管β-淀粉样蛋白(Aβ)沉积物共定位. 实验证实Medin能够与Aβ共同形成异源纤维, 并通过交叉接种机制调控Aβ的聚集. 然而, Medin与Aβ共聚集的微观机制仍不清楚. 本文利用大规模的全原子副本交换分子动力学模拟(累计模拟时间72 μs), 对Aβ42与Medin三聚体在不同多肽环境(即自聚集vs.共聚集)的相互作用及构象分布进行了研究. 结果表明, Aβ42与Medin的亲和力更高, Aβ42与Medin在自身或彼此结合时具有相似的分子识别位点或区域, 为共聚集提供基础. Aβ42的N端与Medin的C端在Aβ42-Medin交叉聚集中起到关键作用. 更重要的是, 共聚集显著地改变了Aβ42与Medin的相互作用强度、方式以及结构特征. Aβ42-Medin三聚体中, Aβ42分子间相互作用减弱, 仅保留疏水核心区域(16KLVFFA21)之间的结合而提高其他区域的自由度; Medin形成更多的β结构与更少的helix结构, 但Aβ42却形成更多的helix与更少的β; 而Medin中高β倾向性区域向肽链中部和C端迁移, 表明Medin可能通过C端形成β结构作为核心从而驱动其与Aβ42的协同聚集. 本工作在原子水平上详尽地阐明了共聚集对Aβ42与Medin相互作用与结构特征的影响, 为理解Aβ42-Medin共聚集分子机制以及不同疾病之间交叉关联的病理机制提供了有益见解.The aggregation of Medin is closely related to the arterial wall degeneration and cerebrovascular dysfunction. In patients with vascular dementia or Alzheimer’s disease, the concentration of medin in cerebral arterioles increases, and Medin is co-localized with vascular amyloid-β (Aβ) deposits. Previous study demonstrates that Medin interacts directly with Aβ, forming heterologous fibrils with Aβ and promoting Aβ aggregation. However, the basic mechanism of the co-aggregation between Medin and Aβ remains largely elusive. Here, we explore the interactions and conformational ensembles of Aβ42/Medin trimers in different peptide environments (self-aggregation vs. co-aggregation) by performing all-atom replica exchange molecular dynamic simulation on Aβ42/Medin homotrimers and Aβ42-Medin heterotrimer with an accumulated simulation time of 72 μs. Our results reveal that Aβ42 exhibits higher affinity with Medin, and Aβ42 and Medin have similar molecular recognition sites in self-aggregation and co-aggregation. The N-terminus of Aβ42 and the C-terminus of Medin play critical roles in Aβ42-Medin cross-talk. More importantly, co-aggregation significantly changes the interaction strength, binding patterns and structural characteristics of Aβ42 and Medin. Intermolecular interactions of Aβ42 trimers are relatively weak among three trimers, and the binding sites are concentrated between N- and N-termini, between N- and C-termini, and between C- and C-termini of Aβ42. In contrast, intermolecular interactions of Medin trimers are the strongest, and the binding sites are widely and uniformly distributed in Medin peptides. Intermolecular interactions of Aβ42 in Aβ42-Medin heterotrimer decrease compared with those of Aβ42 trimers, only the binding of the hydrophobic core regions (16KLVFFA21) is retained and other regions of Aβ42 gain increase flexibility. Two-dimensional free energy landscapes reveal distinct conformational diversities between the homo- and heterotrimers, with the order of diversity being Medin/Aβ42-Medin trimers > Aβ42 trimers. The Rg of Aβ42 trimers is smaller than those of the other two trimers, implying that Aβ42 trimers possess a more compact structure, whereas Medin/Aβ42-Medin trimers exhibit a relatively loose conformation. The Aβ42 trimers possess the highest β content whereas Medin trimers exhibit the lowest β probability. It is found that Aβ42-Medin co-aggregation induces Medin to form more β-structures with longer lengths and fewer helices, while promoting Aβ42 to form more helices and fewer β-structures. High β-propensity regions of Medin in heterotrimers shift towards the C-terminus of Medin, suggesting that Medin utilizes its C-terminal β region as a core motif to drive its co-aggregation with Aβ42. These results elucidate the detailed influences of co-aggregation on the interactions and conformations of Aβ42 and Medin. This work provides key insights into the molecular mechanism of Aβ42-Medin co-aggregation and the pathological mechanisms of cross-linking between related diseases.
-
Keywords:
- peptide co-aggregation /
- amyloid-β /
- Medin /
- molecular dynamic simulations
[1] Giordano X, Fernandez M C 2023 Alzheimers Dementia. 19 e075955
 Google Scholar
Google Scholar
[2] Scheltens P, De Strooper B, Kivipelto M, Holstege H, Chételat G, Teunissen C E, Cummings J, van der Flier W M 2021 Lancet 397 1577
 Google Scholar
Google Scholar
[3] Li X, Yang Z, Chen Y, Zhang S, Wei G, Zhang L 2023 J. Phys. Chem. B 127 4050
 Google Scholar
Google Scholar
[4] Ball K A, Phillips A H, Wemmer D E, Head-Gordon T 2013 Biophys. J. 104 2714
 Google Scholar
Google Scholar
[5] Li X, Zhang Y, Wang Y, Zhang S, Zhang L 2024 J. Phys. Chem. B 128 1843
 Google Scholar
Google Scholar
[6] Nunan J, Small D H 2000 FEBS Lett. 483 6
 Google Scholar
Google Scholar
[7] Huang Y, Potter R, Sigurdson W, Santacruz A, Shih S, Ju Y E, Kasten T, Morris J C, Mintun M, Duntley S, Bateman R J 2012 Arch. Neurol. 69 51
 Google Scholar
Google Scholar
[8] Häggqvist B, Näslund J, Sletten K, Westermark G T, Mucchiano G, Tjernberg L O, Nordstedt C, Engström U, Westermark P 1999 Proc. Natl. Acad. Sci. U. S. A. 96 8669
 Google Scholar
Google Scholar
[9] Karamanova N, Truran S, Serrano G E, Beach T G, Madine J, Weissig V, Davies H A, Veldhuizen J, Nikkhah M, Hansen M, Zhang W, D'Souza K, Franco D A, Migrino R Q 2020 J. Am. Heart Assoc. 9 e014810
 Google Scholar
Google Scholar
[10] Madine J, Davies H A, Migrino R Q, Ruotsalainen S E, Wagner J, Neher J J 2023 Nat. Aging 3 1039
 Google Scholar
Google Scholar
[11] Larsson A, Söderberg L, Westermark G T, Sletten K, Engström U, Tjernberg L O, Näslund J, Westermark P 2007 Biochem. Biophys. Res. Commun. 361 822
 Google Scholar
Google Scholar
[12] Eisenberg D, Jucker M 2012 Cell 148 1188
 Google Scholar
Google Scholar
[13] Iadanza M G, Jackson M P, Hewitt E W, Ranson N A, Radford S E 2018 Nat. Rev. Mol. Cell Biol. 19 755
 Google Scholar
Google Scholar
[14] Ren B, Zhang Y, Zhang M, Liu Y, Zhang D, Gong X, Feng Z, Tang J, Chang Y, Zheng J 2019 J. Mater. Chem. B 7 7267
 Google Scholar
Google Scholar
[15] Zhang Y, Tang Y, Zhang D, Liu Y, He J, Chang Y, Zheng J 2021 Chin. J. Chem. Eng. 30 225
 Google Scholar
Google Scholar
[16] Migrino R Q, Karamanova N, Truran S, Serrano G E, Davies H A, Madine J, Beach T G 2020 Alzheimers Dementia 12 e12072
 Google Scholar
Google Scholar
[17] Tayler H, Miners J S, Güzel Ö, MacLachlan R, Love S 2021 Brain Pathol. 31 e12935
 Google Scholar
Google Scholar
[18] Benson M D, Buxbaum J N, Eisenberg D S, Merlini G, Saraiva M J M, Sekijima Y, Sipe J D, Westermark P 2020 Amyloid 27 217
 Google Scholar
Google Scholar
[19] Jackson R J, Rudinskiy N, Herrmann A G, Croft S, Kim J M, Petrova V, Ramos-Rodriguez J J, Pitstick R, Wegmann S, Garcia-Alloza M, Carlson G A, Hyman B T, Spires-Jones T L 2016 Eur. J. Neurosci. 44 3056
 Google Scholar
Google Scholar
[20] Wagner J, Degenhardt K, Veit M, Louros N, Konstantoulea K, Skodras A, Wild K, Liu P, Obermüller U, Bansal V, Dalmia A, Häsler L M, Lambert M, De Vleeschouwer M, Davies H A, Madine J, Kronenberg-Versteeg D, Feederle R, Del Turco D, Nilsson K P R, Lashley T, Deller T, Gearing M, Walker L C, Heutink P, Rousseau F, Schymkowitz J, Jucker M, Neher J J 2022 Nature 612 123
 Google Scholar
Google Scholar
[21] Huang F, Fan X, Wang Y, Zou Y, Lian J, Wang C, Ding F, Sun Y 2024 Brief. Bioinform. 25 bbad526
 Google Scholar
Google Scholar
[22] Howitz W J, Wierzbicki M, Cabanela R W, Saliba C, Motavalli A, Tran N, Nowick J S 2020 J. Am. Chem. Soc. 142 15870
 Google Scholar
Google Scholar
[23] Davies H A, Madine J, Middleton D A 2015 J. Biol. Chem. 290 7791
 Google Scholar
Google Scholar
[24] Davies H A, Rigden D J, Phelan M M, Madine J 2017 Sci. Rep. 7 45224
 Google Scholar
Google Scholar
[25] Huang F, Yan J, Zhang X, Xu H, Lian J, Yang X, Wang C, Ding F, Sun Y 2024 Colloids Surf. , B 244 114192
 Google Scholar
Google Scholar
[26] Huang F, Fan X, Wang Y, Wang C, Zou Y, Lian J, Ding F, Sun Y 2023 J. Chem. Inf. Model. 63 6376
 Google Scholar
Google Scholar
[27] 徐成, 林召, 杨恺, 元冰 2020 69 108701
 Google Scholar
Google Scholar
Xu C, Lin Z, Yang K, Yuan B 2020 Acta Phys. Sin. 69 108701
 Google Scholar
Google Scholar
[28] 王康, 徐成, 吴晋锋, 杨恺, 元冰 2021 70 178701
 Google Scholar
Google Scholar
Wang K, Xu C, Wu J F, Yang K, Yuan B 2021 Acta Phys. Sin. 70 178701
 Google Scholar
Google Scholar
[29] 谭金鹏, 张婉婷, 徐成, 卢雪梅, 朱文圣, 杨恺, 元冰 2024 73 188702
 Google Scholar
Google Scholar
Tan J P, Zhang W T, Xu C, Lu X M, Zhu W S, Yang K, Yuan B 2024 Acta Phys. Sin. 73 188702
 Google Scholar
Google Scholar
[30] Tu W, Dong X, Ou L, Zhang X, Yuan B, Yang K 2023 Chem. Res. Chin. Univ. 39 829
 Google Scholar
Google Scholar
[31] Lao Z, Tang Y, Dong X, Tan Y, Li X, Liu X, Li L, Guo C, Wei G 2024 Nanoscale 16 4025
 Google Scholar
Google Scholar
[32] Liu X, Lao Z, Li X, Dong X, Wei G 2022 Phys. Chem. Chem. Phys. 24 16263
 Google Scholar
Google Scholar
[33] Qi R, Wei G, Ma B, Nussinov R 2018 Methods Mol. Biol. 1777 101
 Google Scholar
Google Scholar
[34] Sugita Y, Okamoto Y 1999 Chem. Phys. Lett. 314 141
 Google Scholar
Google Scholar
[35] Miron R A, Fichthorn K A 2003 J. Chem. Phys. 119 6210
 Google Scholar
Google Scholar
[36] Kästner J 2011 Wiley Interdiscip. Rev. Comput. Mol. Sci. 1 932
 Google Scholar
Google Scholar
[37] Qi R, Luo Y, Wei G, Nussinov R, Ma B 2015 J. Phys. Chem. Lett. 6 3276
 Google Scholar
Google Scholar
[38] Dong X, Bera S, Qiao Q, Tang Y, Lao Z, Luo Y, Gazit E, Wei G 2021 J. Phys. Chem. Lett. 12 2576
 Google Scholar
Google Scholar
[39] Li X, Chen Y, Yang Z, Zhang S, Wei G, Zhang L 2024 Int. J. Biol. Macromol. 254 127841
 Google Scholar
Google Scholar
[40] Tan Y, Chen Y, Liu X, Tang Y, Lao Z, Wei G 2023 Int. J. Biol. Macromol. 241 124659
 Google Scholar
Google Scholar
[41] Lao Z, Dong X, Liu X, Li F, Chen Y, Tang Y, Wei G 2022 J. Chem. Inf. Model. 62 3227
 Google Scholar
Google Scholar
[42] Dong X, Qi R, Qiao Q, Li X, Li F, Wan J, Zhang Q, Wei G 2021 Phys. Chem. Chem. Phys. 23 20406
 Google Scholar
Google Scholar
[43] Mo Y, Brahmachari S, Lei J, Gilead S, Tang Y, Gazit E, Wei G 2018 ACS Chem. Neurosci. 9 2741
 Google Scholar
Google Scholar
[44] Guo C, Côté S, Mousseau N, Wei G 2015 J. Phys. Chem. B 119 3366
 Google Scholar
Google Scholar
[45] Gremer L, Schölzel D, Schenk C, Reinartz E, Labahn J, Ravelli R B G, Tusche M, Lopez-Iglesias C, Hoyer W, Heise H, Willbold D, Schröder G F 2017 Science 358 116
 Google Scholar
Google Scholar
[46] Jumper J, Evans R, Pritzel A, Green T, Figurnov M, Ronneberger O, Tunyasuvunakool K, Bates R, Žídek A, Potapenko A, Bridgland A, Meyer C, Kohl S A A, Ballard A J, Cowie A, Romera-Paredes B, Nikolov S, Jain R, Adler J, Back T, Petersen S, Reiman D, Clancy E, Zielinski M, Steinegger M, Pacholska M, Berghammer T, Bodenstein S, Silver D, Vinyals O, Senior A W, Kavukcuoglu K, Kohli P, Hassabis D 2021 Nature 596 583
 Google Scholar
Google Scholar
[47] Humphrey W, Dalke A, Schulten K 1996 J. Mol. Graphics 14 33
 Google Scholar
Google Scholar
[48] Abraham M J, Murtola T, Schulz R, Páll S, Smith J C, Hess B, Lindahl E 2015 SoftwareX 1–2 19
 Google Scholar
Google Scholar
[49] Lindorff-Larsen K, Piana S, Palmo K, Maragakis P, Klepeis J L, Dror R O, Shaw D E 2010 Proteins 78 1950
 Google Scholar
Google Scholar
[50] Chen Y, Li X, Zhan C, Lao Z, Li F, Dong X, Wei G 2021 ACS Chem. Neurosci. 12 4007
 Google Scholar
Google Scholar
[51] Lopes P E, Guvench O, MacKerell Jr. A D 2015 Methods Mol. Biol. 1215 47
 Google Scholar
Google Scholar
[52] Tan Y, Chen Y, Pan T, Tang Y, Liu X, Yu Y, Wei G 2025 J. Chem. Inf. Model. 65 4643
 Google Scholar
Google Scholar
[53] Wang W 2021 Phys. Chem. Chem. Phys. 23 777
 Google Scholar
Google Scholar
[54] Best R B, Zheng W, Mittal J 2014 J. Chem. Theory Comput. 10 5113
 Google Scholar
Google Scholar
[55] Huang J, Rauscher S, Nawrocki G, Ran T, Feig M, de Groot B L, Grubmüller H, MacKerell A D, Jr. 2017 Nat. Methods 14 71
 Google Scholar
Google Scholar
[56] Piana S, Donchev A G, Robustelli P, Shaw D E 2015 J. Phys. Chem. B 119 5113
 Google Scholar
Google Scholar
[57] Zerze G H, Zheng W, Best R B, Mittal J 2019 J. Phys. Chem. Lett. 10 2227
 Google Scholar
Google Scholar
[58] Parrinello M, Rahman A 1981 J. Appl. Phys. 52 7182
 Google Scholar
Google Scholar
[59] Bussi G, Donadio D, Parrinello M 2007 J. Chem. Phys. 126 014101
 Google Scholar
Google Scholar
[60] Li M, Johnson W L, Goddard W A 1992 MRS Online Proc. Lib. 291 285
 Google Scholar
Google Scholar
[61] Miyamoto S, Kollman P A 1992 J. Comput. Chem. 13 952
 Google Scholar
Google Scholar
[62] Hess B 2008 J. Chem. Theory Comput. 4 116
 Google Scholar
Google Scholar
[63] Kabsch W, Sander C 1983 Biopolymers 22 2577
 Google Scholar
Google Scholar
[64] Daura X, Gademann K, Jaun B, Seebach D, van Gunsteren W F, Mark A E 1999 Angew. Chem. Int. Ed. 38 236
 Google Scholar
Google Scholar
[65] Zhang Y, Liu Y, Zhao W, Sun Y 2021 Int. J. Biol. Macromol. 193 1
 Google Scholar
Google Scholar
[66] Li X, Lao Z, Zou Y, Dong X, Li L, Wei G 2021 J. Phys. Chem. B 125 2050
 Google Scholar
Google Scholar
[67] Delano W L http://pymol.org [2025-5-10]
[68] Rigsby R E, Parker A B 2016 Biochem. Mol. Biol. Educ. 44 433
 Google Scholar
Google Scholar
[69] Okumura H, Itoh S G 2022 Molecules 27 2483
 Google Scholar
Google Scholar
[70] Reches M, Gazit E 2004 Amyloid 11 81
 Google Scholar
Google Scholar
[71] Madine J, Copland A, Serpell L C, Middleton D A 2009 Biochemistry 48 3089
 Google Scholar
Google Scholar
-
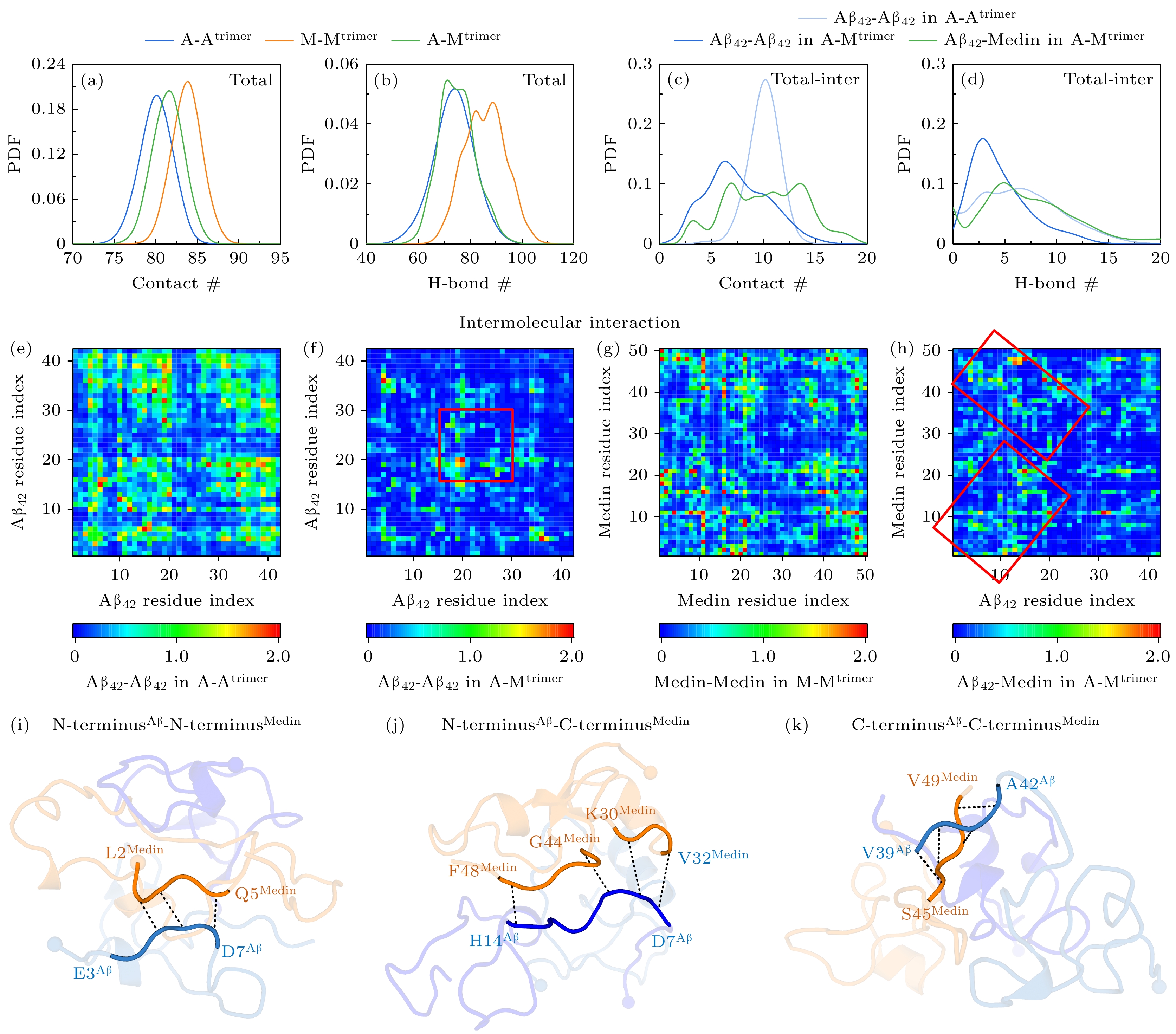
图 1 Aβ42/Medin同源三聚体与Aβ42-Medin异源三聚体的相互作用分析 (a), (b)三个体系中多肽的(a)接触数与(b)氢键数的概率密度分布(probability density function, PDF); (c), (d) Aβ42与Aβ42-Medin三聚体体系中, Aβ42-Aβ42、Aβ42-Medin之间的(c)接触数与(d)氢键数的PDF; (e)—(h)多肽分子间的残基-残基接触作用数图, 即(e) Aβ42三聚体中Aβ42-Aβ42的残基-残基接触数图、(f) Aβ42-Medin三聚体中Aβ42-Aβ42的残基-残基接触数图、(g) Medin三聚体中Medin-Medin的残基-残基接触数图、(h) Aβ42-Medin三聚体中Aβ42-Medin的残基-残基接触数图; (i)—(k)展示Aβ42与Medin结合区域的代表性结构快照. 多肽以cartoon方式呈现. Aβ42与Medin结合区域以蓝色和橘色突出显示, 并将区域两端对应的氨基酸标记在结构上
Fig. 1. Analysis of interactions in Aβ42/Medin homotrimers and Aβ42-Medin heterotrimer: (a), (b) Probability density function (PDF) of (a) contact number and (b) hydrogen-bond (H-bond) number of peptides in three systems; (c), (d) PDF of (c) contact number and (d) H-bond number between Aβ42 and Aβ42 as well as between Aβ42 and Medin in A-Atrimer and A-Mtrimer systems; (e)–(h) 2D residue-residue contact maps of intermolecular interactions for (e) Aβ42-Aβ42 in A-Atrimer, (f) Aβ42-Aβ42 in A-Mtrimer, (g) Medin-Medin in M-Mtrimer and (h) Aβ42-Medin in A-Mtrimer; (i)–(k) representative snapshots illustrate the binding regions between Aβ42 and Medin in A-Mtrimer system.
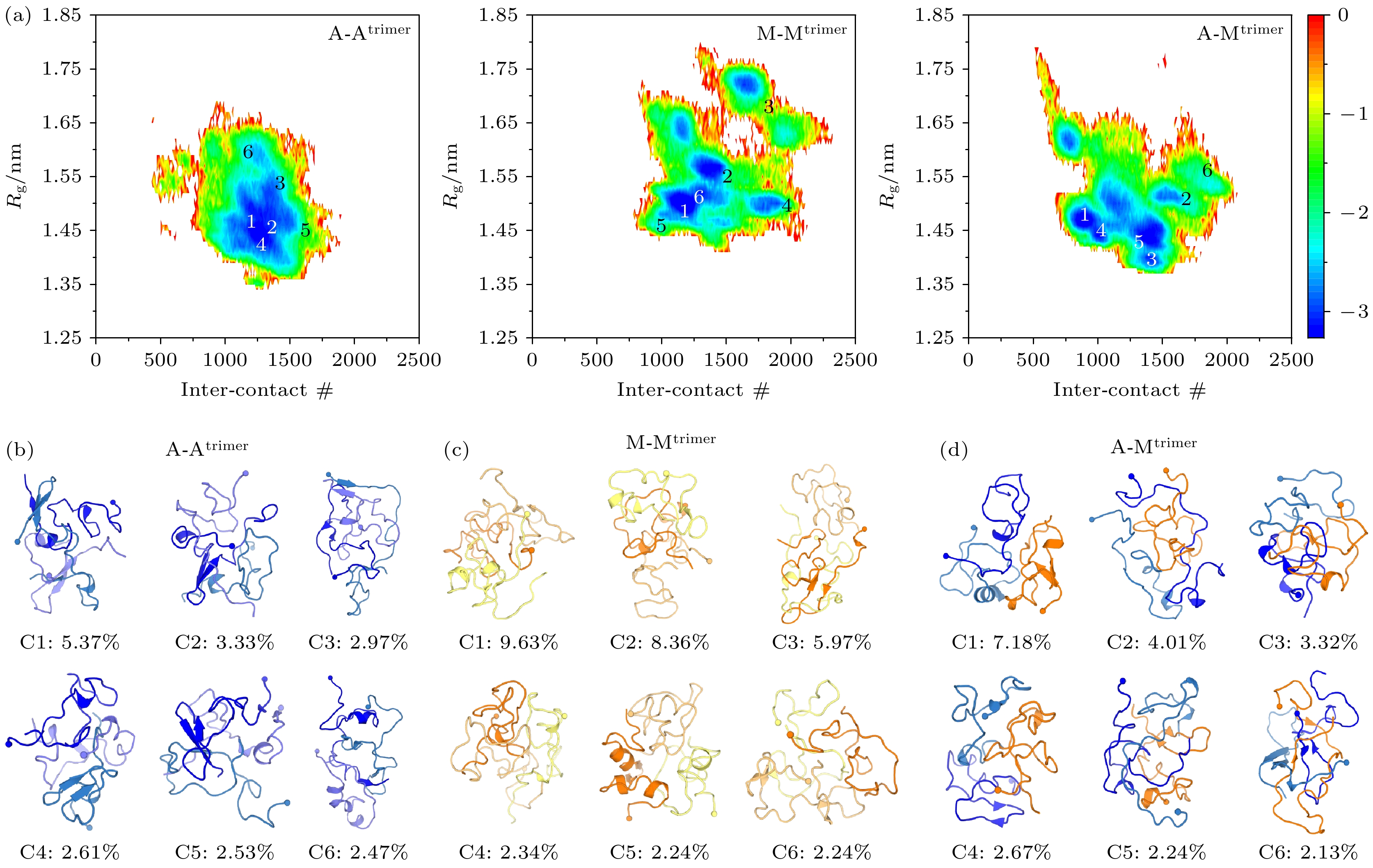
图 2 Aβ42与Medin在同源与异源三聚体体系中的构象特征分析 (a)以链间接触数和回转半径为反应坐标的二维构象FELs: A-Atrimer体系(左)、M-Mtrimer体系(中)、A-Mtrimer体系(右); (b)—(d)聚类分析中概率最高的六个聚类构象簇(C1—C6)的中心结构及每个聚类对应的概率(标记在对应中心结构下方): (b) Aβ42三聚体、(c) Medin三聚体以及(d) Aβ42-Medin三聚体. 这些聚类中心结构的位置标记在二维自由能图景(a)中. 多肽以cartoon方式呈现, 每条多肽链N端残基的Cα原子以小球标记. Aβ42和Medin分别用橙色系和蓝色系颜色显示
Fig. 2. Analysis of conformational characteristics of Aβ42/Medin homotrimers and Aβ42-Medin heterotrimer: (a) FELs as a function of intermolecular contact number and trimeric Rg in A-Atrimer (left), M-Mtrimer (middle) and A-Mtrimer (right) systems; (b)–(d) representative conformations for the six most-populated clusters (C1–C6) along with their corresponding populations (marked below the corresponding representative conformations) of (b) Aβ42 trimer, (c) Medin trimer and (d) Aβ42-Medin trimer. The locations of those representative conformations are labeled on the FEL plots. Aβ42 and Medin are shown in cartoon, with the N-terminal Cα atom of each chain represented by a sphere. Aβ42 and Medin peptides are colored in blue and orange, respectively.
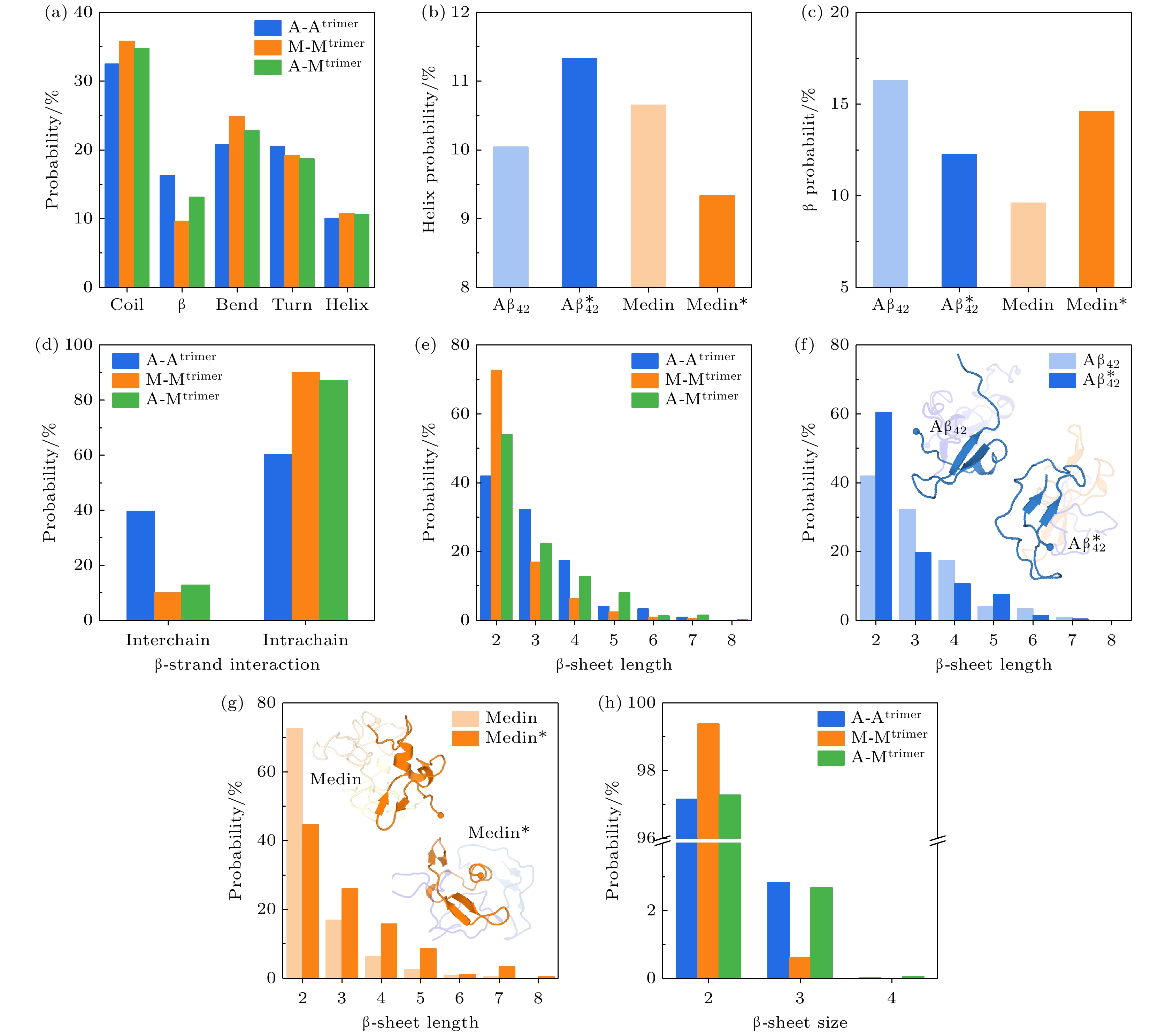
图 3 Aβ42与Medin在同源与异源三聚体体系中的二级结构分析 (a)三个体系中的各种二级结构的概率统计; (b) Aβ42与Medin在不同体系中形成helix结构的概率: A-Atrimer和A-Mtrimer体系中的Aβ42 (浅蓝vs.深蓝)以及M-Mtrimer和A-Mtrimer体系中的Medin (浅橙色vs.深橙色), 其中异源聚集体体系用*号表示; (c) Aβ42与Medin在不同体系中形成β结构的概率; (d) β结构以链间和链内方式进行排列的概率; (e)三个体系的三聚体中β-sheet长度的概率分布; (f) A-Atrimer和A-Mtrimer (*) 体系中Aβ42的β-sheet长度的概率分布; (g) M-Mtrimer和A-Mtrimer (*) 体系中Medin的β-sheet长度的概率分布; (h)三个体系的三聚体中β-sheet尺寸的概率分布
Fig. 3. Analysis of secondary structures of Aβ42/Medin homotrimers and Aβ42-Medin heterotrimer: (a) Each secondary structure probability of Aβ42 homotrimer, Medin homotrimer and Aβ42-Medin heterotrimer; (b) helix probability of Aβ42 and Medin in different systems: Aβ42 in A-Atrimer system (light blue) vs. Aβ42 in A-Mtrimer system (*, blue) and Medin in M-Mtrimer system (light orange) vs. Medin in A-Mtrimer system (*, orange); (c) β probability of Aβ42 and Medin in different systems; (d) probability of β arrangement with interchain and intrachain manners; (e) probability of β-sheet length in three systems; (f) probability of β-sheet length of Aβ42 in A-Atrimer and A-Mtrimer systems; (g) probability of β-sheet length of Medin in M-Mtrimer and A-Mtrimer systems; (h) probability of β-sheet size in three systems.
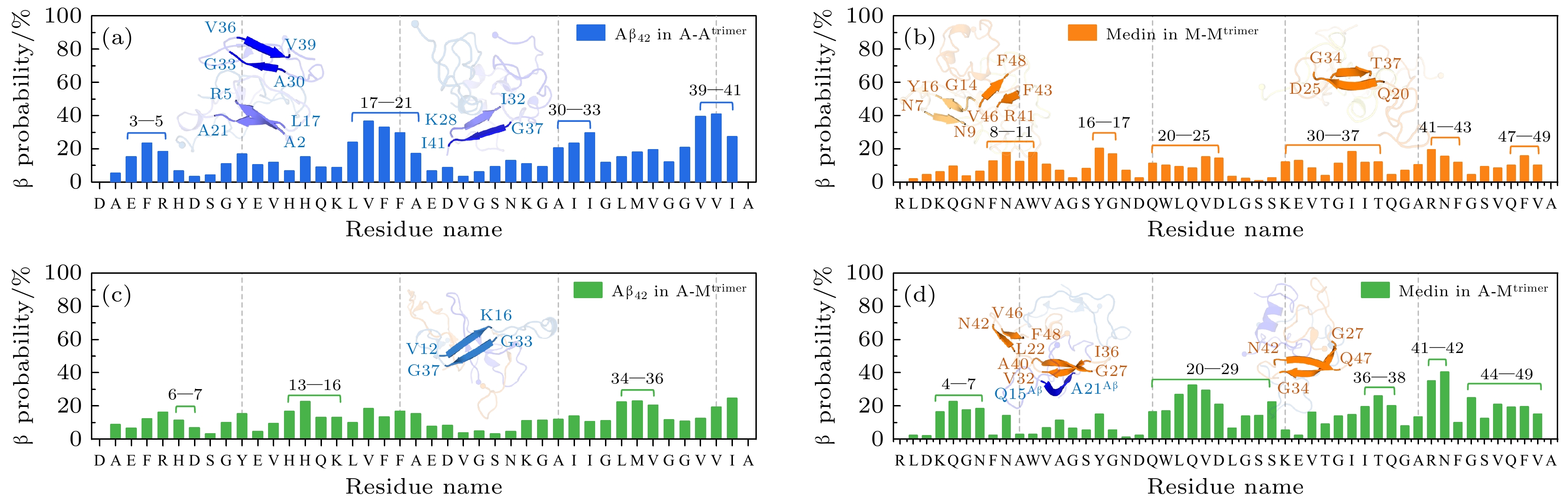
图 4 β结构概率和β结构形成区域的分析 (a), (c) A-Atrimer和A-Mtrimer体系中Aβ42的每个氨基酸形成β结构的概率; (b), (d) M-Mtrimer和A-Mtrimer体系中Medin的每个氨基酸形成β结构的概率. 富含β-sheet结构的代表性Aβ42, Medin和Aβ42-Medin三聚体构象展示在对应的残基-β结构概率图中. 多肽采用cartoon表示形式, Aβ42与Medin中的β结构区域以蓝色和橙色突出显示
Fig. 4. β-sheet probability and β-sheet formation regions of Aβ42 and Medin in homotrimer and heterotrimer. Residue-based β probability distribution of Aβ42 and Medin peptides respectively in (a) Aβ42 homotrimer/(b) Medin homotrimer and (c), (d) Aβ42-Medin heterotrimer. Representative β-sheet-rich conformations of Aβ42, Medin and Aβ42-Medin trimers are illustrated as insets in (a)–(d). Aβ42 and Medin peptides are shown in cartoon with the β-sheet-rich regions highlighted in blue and orange, respectively.
-
[1] Giordano X, Fernandez M C 2023 Alzheimers Dementia. 19 e075955
 Google Scholar
Google Scholar
[2] Scheltens P, De Strooper B, Kivipelto M, Holstege H, Chételat G, Teunissen C E, Cummings J, van der Flier W M 2021 Lancet 397 1577
 Google Scholar
Google Scholar
[3] Li X, Yang Z, Chen Y, Zhang S, Wei G, Zhang L 2023 J. Phys. Chem. B 127 4050
 Google Scholar
Google Scholar
[4] Ball K A, Phillips A H, Wemmer D E, Head-Gordon T 2013 Biophys. J. 104 2714
 Google Scholar
Google Scholar
[5] Li X, Zhang Y, Wang Y, Zhang S, Zhang L 2024 J. Phys. Chem. B 128 1843
 Google Scholar
Google Scholar
[6] Nunan J, Small D H 2000 FEBS Lett. 483 6
 Google Scholar
Google Scholar
[7] Huang Y, Potter R, Sigurdson W, Santacruz A, Shih S, Ju Y E, Kasten T, Morris J C, Mintun M, Duntley S, Bateman R J 2012 Arch. Neurol. 69 51
 Google Scholar
Google Scholar
[8] Häggqvist B, Näslund J, Sletten K, Westermark G T, Mucchiano G, Tjernberg L O, Nordstedt C, Engström U, Westermark P 1999 Proc. Natl. Acad. Sci. U. S. A. 96 8669
 Google Scholar
Google Scholar
[9] Karamanova N, Truran S, Serrano G E, Beach T G, Madine J, Weissig V, Davies H A, Veldhuizen J, Nikkhah M, Hansen M, Zhang W, D'Souza K, Franco D A, Migrino R Q 2020 J. Am. Heart Assoc. 9 e014810
 Google Scholar
Google Scholar
[10] Madine J, Davies H A, Migrino R Q, Ruotsalainen S E, Wagner J, Neher J J 2023 Nat. Aging 3 1039
 Google Scholar
Google Scholar
[11] Larsson A, Söderberg L, Westermark G T, Sletten K, Engström U, Tjernberg L O, Näslund J, Westermark P 2007 Biochem. Biophys. Res. Commun. 361 822
 Google Scholar
Google Scholar
[12] Eisenberg D, Jucker M 2012 Cell 148 1188
 Google Scholar
Google Scholar
[13] Iadanza M G, Jackson M P, Hewitt E W, Ranson N A, Radford S E 2018 Nat. Rev. Mol. Cell Biol. 19 755
 Google Scholar
Google Scholar
[14] Ren B, Zhang Y, Zhang M, Liu Y, Zhang D, Gong X, Feng Z, Tang J, Chang Y, Zheng J 2019 J. Mater. Chem. B 7 7267
 Google Scholar
Google Scholar
[15] Zhang Y, Tang Y, Zhang D, Liu Y, He J, Chang Y, Zheng J 2021 Chin. J. Chem. Eng. 30 225
 Google Scholar
Google Scholar
[16] Migrino R Q, Karamanova N, Truran S, Serrano G E, Davies H A, Madine J, Beach T G 2020 Alzheimers Dementia 12 e12072
 Google Scholar
Google Scholar
[17] Tayler H, Miners J S, Güzel Ö, MacLachlan R, Love S 2021 Brain Pathol. 31 e12935
 Google Scholar
Google Scholar
[18] Benson M D, Buxbaum J N, Eisenberg D S, Merlini G, Saraiva M J M, Sekijima Y, Sipe J D, Westermark P 2020 Amyloid 27 217
 Google Scholar
Google Scholar
[19] Jackson R J, Rudinskiy N, Herrmann A G, Croft S, Kim J M, Petrova V, Ramos-Rodriguez J J, Pitstick R, Wegmann S, Garcia-Alloza M, Carlson G A, Hyman B T, Spires-Jones T L 2016 Eur. J. Neurosci. 44 3056
 Google Scholar
Google Scholar
[20] Wagner J, Degenhardt K, Veit M, Louros N, Konstantoulea K, Skodras A, Wild K, Liu P, Obermüller U, Bansal V, Dalmia A, Häsler L M, Lambert M, De Vleeschouwer M, Davies H A, Madine J, Kronenberg-Versteeg D, Feederle R, Del Turco D, Nilsson K P R, Lashley T, Deller T, Gearing M, Walker L C, Heutink P, Rousseau F, Schymkowitz J, Jucker M, Neher J J 2022 Nature 612 123
 Google Scholar
Google Scholar
[21] Huang F, Fan X, Wang Y, Zou Y, Lian J, Wang C, Ding F, Sun Y 2024 Brief. Bioinform. 25 bbad526
 Google Scholar
Google Scholar
[22] Howitz W J, Wierzbicki M, Cabanela R W, Saliba C, Motavalli A, Tran N, Nowick J S 2020 J. Am. Chem. Soc. 142 15870
 Google Scholar
Google Scholar
[23] Davies H A, Madine J, Middleton D A 2015 J. Biol. Chem. 290 7791
 Google Scholar
Google Scholar
[24] Davies H A, Rigden D J, Phelan M M, Madine J 2017 Sci. Rep. 7 45224
 Google Scholar
Google Scholar
[25] Huang F, Yan J, Zhang X, Xu H, Lian J, Yang X, Wang C, Ding F, Sun Y 2024 Colloids Surf. , B 244 114192
 Google Scholar
Google Scholar
[26] Huang F, Fan X, Wang Y, Wang C, Zou Y, Lian J, Ding F, Sun Y 2023 J. Chem. Inf. Model. 63 6376
 Google Scholar
Google Scholar
[27] 徐成, 林召, 杨恺, 元冰 2020 69 108701
 Google Scholar
Google Scholar
Xu C, Lin Z, Yang K, Yuan B 2020 Acta Phys. Sin. 69 108701
 Google Scholar
Google Scholar
[28] 王康, 徐成, 吴晋锋, 杨恺, 元冰 2021 70 178701
 Google Scholar
Google Scholar
Wang K, Xu C, Wu J F, Yang K, Yuan B 2021 Acta Phys. Sin. 70 178701
 Google Scholar
Google Scholar
[29] 谭金鹏, 张婉婷, 徐成, 卢雪梅, 朱文圣, 杨恺, 元冰 2024 73 188702
 Google Scholar
Google Scholar
Tan J P, Zhang W T, Xu C, Lu X M, Zhu W S, Yang K, Yuan B 2024 Acta Phys. Sin. 73 188702
 Google Scholar
Google Scholar
[30] Tu W, Dong X, Ou L, Zhang X, Yuan B, Yang K 2023 Chem. Res. Chin. Univ. 39 829
 Google Scholar
Google Scholar
[31] Lao Z, Tang Y, Dong X, Tan Y, Li X, Liu X, Li L, Guo C, Wei G 2024 Nanoscale 16 4025
 Google Scholar
Google Scholar
[32] Liu X, Lao Z, Li X, Dong X, Wei G 2022 Phys. Chem. Chem. Phys. 24 16263
 Google Scholar
Google Scholar
[33] Qi R, Wei G, Ma B, Nussinov R 2018 Methods Mol. Biol. 1777 101
 Google Scholar
Google Scholar
[34] Sugita Y, Okamoto Y 1999 Chem. Phys. Lett. 314 141
 Google Scholar
Google Scholar
[35] Miron R A, Fichthorn K A 2003 J. Chem. Phys. 119 6210
 Google Scholar
Google Scholar
[36] Kästner J 2011 Wiley Interdiscip. Rev. Comput. Mol. Sci. 1 932
 Google Scholar
Google Scholar
[37] Qi R, Luo Y, Wei G, Nussinov R, Ma B 2015 J. Phys. Chem. Lett. 6 3276
 Google Scholar
Google Scholar
[38] Dong X, Bera S, Qiao Q, Tang Y, Lao Z, Luo Y, Gazit E, Wei G 2021 J. Phys. Chem. Lett. 12 2576
 Google Scholar
Google Scholar
[39] Li X, Chen Y, Yang Z, Zhang S, Wei G, Zhang L 2024 Int. J. Biol. Macromol. 254 127841
 Google Scholar
Google Scholar
[40] Tan Y, Chen Y, Liu X, Tang Y, Lao Z, Wei G 2023 Int. J. Biol. Macromol. 241 124659
 Google Scholar
Google Scholar
[41] Lao Z, Dong X, Liu X, Li F, Chen Y, Tang Y, Wei G 2022 J. Chem. Inf. Model. 62 3227
 Google Scholar
Google Scholar
[42] Dong X, Qi R, Qiao Q, Li X, Li F, Wan J, Zhang Q, Wei G 2021 Phys. Chem. Chem. Phys. 23 20406
 Google Scholar
Google Scholar
[43] Mo Y, Brahmachari S, Lei J, Gilead S, Tang Y, Gazit E, Wei G 2018 ACS Chem. Neurosci. 9 2741
 Google Scholar
Google Scholar
[44] Guo C, Côté S, Mousseau N, Wei G 2015 J. Phys. Chem. B 119 3366
 Google Scholar
Google Scholar
[45] Gremer L, Schölzel D, Schenk C, Reinartz E, Labahn J, Ravelli R B G, Tusche M, Lopez-Iglesias C, Hoyer W, Heise H, Willbold D, Schröder G F 2017 Science 358 116
 Google Scholar
Google Scholar
[46] Jumper J, Evans R, Pritzel A, Green T, Figurnov M, Ronneberger O, Tunyasuvunakool K, Bates R, Žídek A, Potapenko A, Bridgland A, Meyer C, Kohl S A A, Ballard A J, Cowie A, Romera-Paredes B, Nikolov S, Jain R, Adler J, Back T, Petersen S, Reiman D, Clancy E, Zielinski M, Steinegger M, Pacholska M, Berghammer T, Bodenstein S, Silver D, Vinyals O, Senior A W, Kavukcuoglu K, Kohli P, Hassabis D 2021 Nature 596 583
 Google Scholar
Google Scholar
[47] Humphrey W, Dalke A, Schulten K 1996 J. Mol. Graphics 14 33
 Google Scholar
Google Scholar
[48] Abraham M J, Murtola T, Schulz R, Páll S, Smith J C, Hess B, Lindahl E 2015 SoftwareX 1–2 19
 Google Scholar
Google Scholar
[49] Lindorff-Larsen K, Piana S, Palmo K, Maragakis P, Klepeis J L, Dror R O, Shaw D E 2010 Proteins 78 1950
 Google Scholar
Google Scholar
[50] Chen Y, Li X, Zhan C, Lao Z, Li F, Dong X, Wei G 2021 ACS Chem. Neurosci. 12 4007
 Google Scholar
Google Scholar
[51] Lopes P E, Guvench O, MacKerell Jr. A D 2015 Methods Mol. Biol. 1215 47
 Google Scholar
Google Scholar
[52] Tan Y, Chen Y, Pan T, Tang Y, Liu X, Yu Y, Wei G 2025 J. Chem. Inf. Model. 65 4643
 Google Scholar
Google Scholar
[53] Wang W 2021 Phys. Chem. Chem. Phys. 23 777
 Google Scholar
Google Scholar
[54] Best R B, Zheng W, Mittal J 2014 J. Chem. Theory Comput. 10 5113
 Google Scholar
Google Scholar
[55] Huang J, Rauscher S, Nawrocki G, Ran T, Feig M, de Groot B L, Grubmüller H, MacKerell A D, Jr. 2017 Nat. Methods 14 71
 Google Scholar
Google Scholar
[56] Piana S, Donchev A G, Robustelli P, Shaw D E 2015 J. Phys. Chem. B 119 5113
 Google Scholar
Google Scholar
[57] Zerze G H, Zheng W, Best R B, Mittal J 2019 J. Phys. Chem. Lett. 10 2227
 Google Scholar
Google Scholar
[58] Parrinello M, Rahman A 1981 J. Appl. Phys. 52 7182
 Google Scholar
Google Scholar
[59] Bussi G, Donadio D, Parrinello M 2007 J. Chem. Phys. 126 014101
 Google Scholar
Google Scholar
[60] Li M, Johnson W L, Goddard W A 1992 MRS Online Proc. Lib. 291 285
 Google Scholar
Google Scholar
[61] Miyamoto S, Kollman P A 1992 J. Comput. Chem. 13 952
 Google Scholar
Google Scholar
[62] Hess B 2008 J. Chem. Theory Comput. 4 116
 Google Scholar
Google Scholar
[63] Kabsch W, Sander C 1983 Biopolymers 22 2577
 Google Scholar
Google Scholar
[64] Daura X, Gademann K, Jaun B, Seebach D, van Gunsteren W F, Mark A E 1999 Angew. Chem. Int. Ed. 38 236
 Google Scholar
Google Scholar
[65] Zhang Y, Liu Y, Zhao W, Sun Y 2021 Int. J. Biol. Macromol. 193 1
 Google Scholar
Google Scholar
[66] Li X, Lao Z, Zou Y, Dong X, Li L, Wei G 2021 J. Phys. Chem. B 125 2050
 Google Scholar
Google Scholar
[67] Delano W L http://pymol.org [2025-5-10]
[68] Rigsby R E, Parker A B 2016 Biochem. Mol. Biol. Educ. 44 433
 Google Scholar
Google Scholar
[69] Okumura H, Itoh S G 2022 Molecules 27 2483
 Google Scholar
Google Scholar
[70] Reches M, Gazit E 2004 Amyloid 11 81
 Google Scholar
Google Scholar
[71] Madine J, Copland A, Serpell L C, Middleton D A 2009 Biochemistry 48 3089
 Google Scholar
Google Scholar
计量
- 文章访问数: 554
- PDF下载量: 34
- 被引次数: 0















 下载:
下载: