-
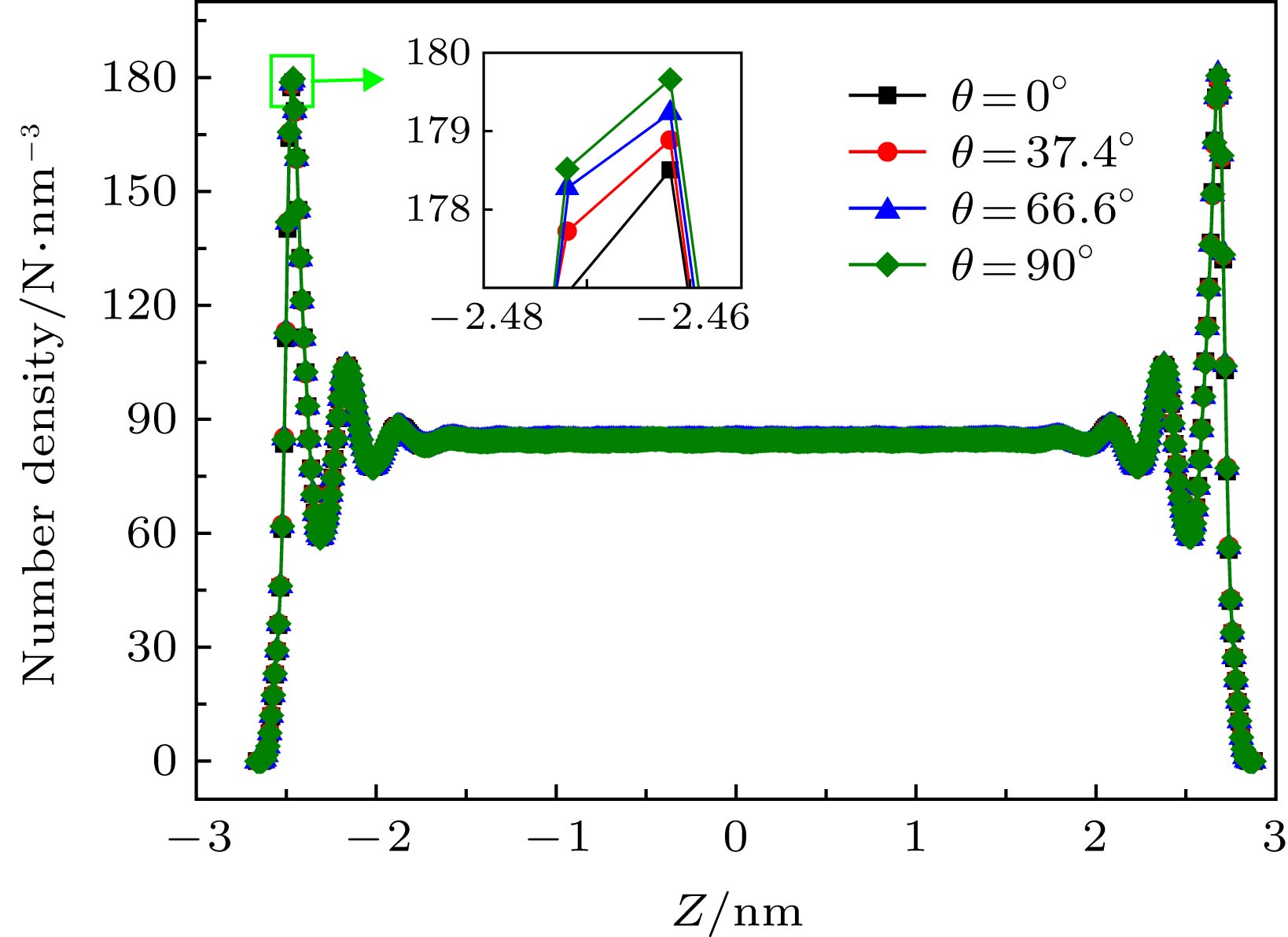
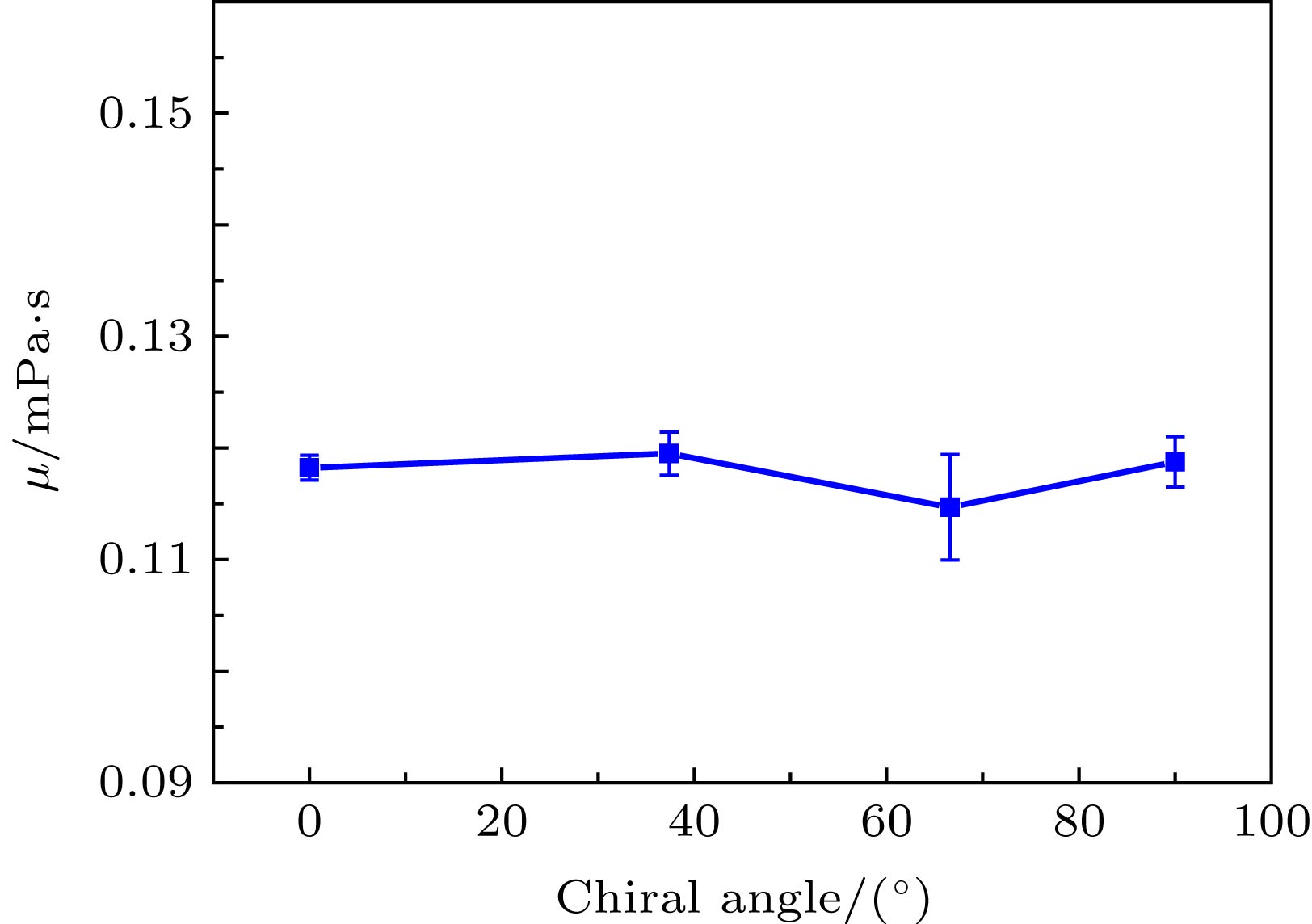
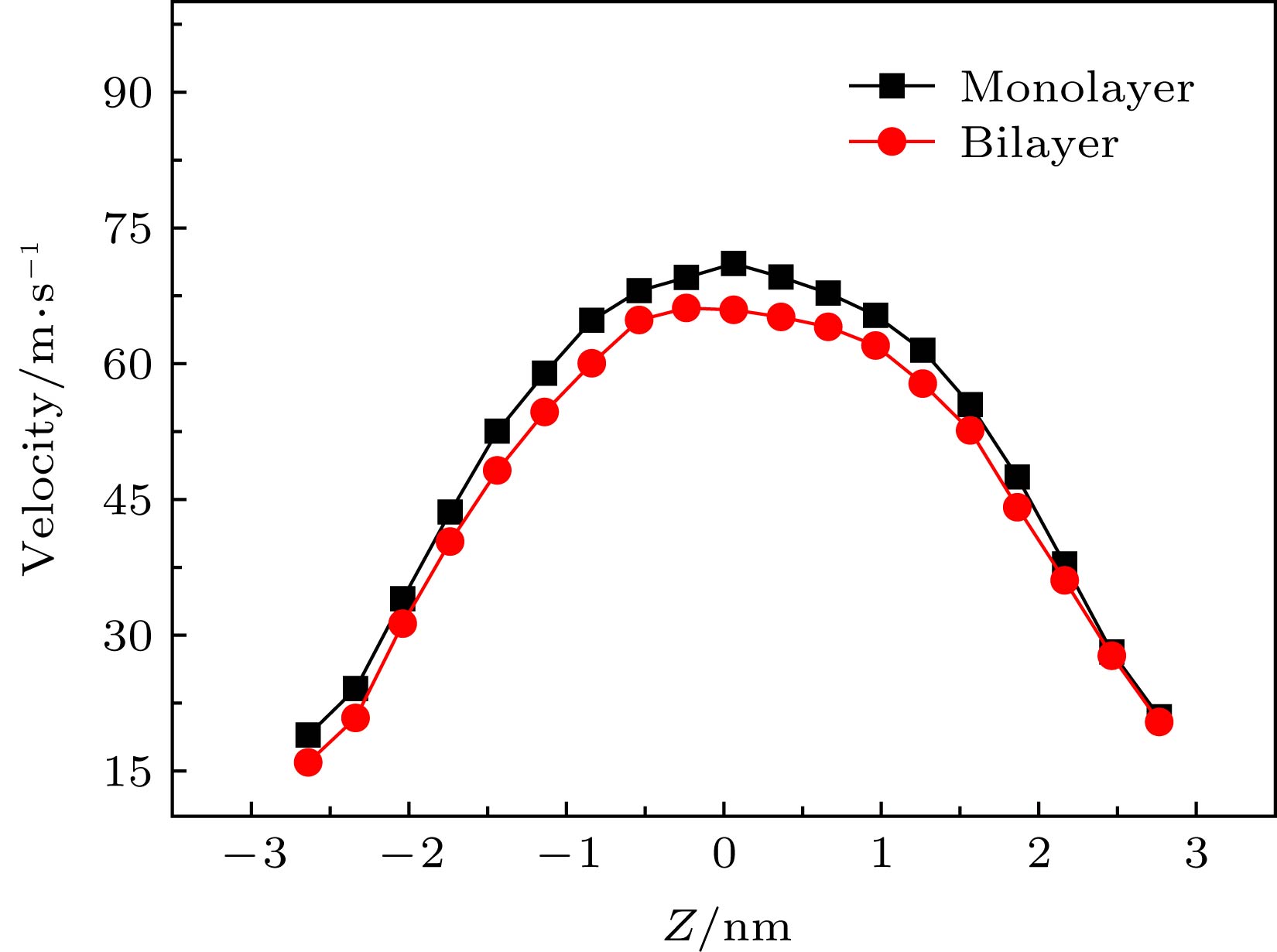
运用分子动力学方法探索了水-黑磷流-固界面各向异性、水流驱动力、黑磷通道宽度和黑磷层数等对黑磷通道内Poiseuille水流流动特性的影响规律. 研究结果表明: 随着驱动力的增加, 边界滑移速度随之增加; 各向异性也会对压力驱动作用下纳米通道内的水分子的流动特性产生影响, 具体表现为边界滑移速度会随着手性角度的增加而减小, 而水分子黏度系数却不受各向异性的影响. 发现黑磷表面天然的褶皱结构所产生的粗糙势能表面, 是导致流固界面各向异性特性的本质原因. 在加速度值保持不变的情况下, 研究纳米通道宽度和黑磷层数对水分子流动特性的影响, 发现随着纳米通道宽度的增加, 水分子滑移速度随之减小; 双层模型中水分子的速度分布与单层模型差异微小, 而随着层数的增加, 黑磷-水流固交互界面能随之增加, 各向异性规律依然保持不变. 研究结果将为水-黑磷流体器件设计与制备提供理论基础.With the rapid development of low-dimensional materials, the opportunity that promotes the development of micro/nano fluid devices, a new low-dimensional material black phosphorus (BP) has attracted wide attention due to its excellent properties, and has been applied to many areas. In this paper, the influences of driving force, water-BP anisotropy, channels’ width and the number of black phosphorus layers on the flow characteristics of water molecules in the nanochannels are studied by molecular dynamics based on the Poiseuille flow model. The results show that the boundary slip velocity increases with the driving force increasing. The anisotropy will also affect the flow characteristics of water molecules in the nanochannel under the pressure driving the Poiseuille flow. Specifically, the boundary slip velocity decreases with the chirality angle increasing, and the viscosity coefficient of water molecules is still not affected by the anisotropy. The natural rippled structure of the BP surface leads to the coarse potential surface, and further results in the anisotropic boundary slip and interfacial friction between water and BP sheets. With the driving acceleration kept constant, the influences of the width of nanochannels and the number of black phosphorus layers on the boundary slip velocity and viscosity coefficient of water molecules are investigated. The results indicate that the slip velocity of water molecules in the nanochannels decreases with the width of the nanochannels increasing. The velocity profile of water molecules in the bilayer model is slightly different from that in the monolayer model. With the number of BP layers increasing, the energy of BP-water solid-liquid interface increases while the anisotropic interfacial property remains unchanged. The results will provide a theoretical basis for the study of the characteristics of the fluid flowing in the black phosphorus nanochannels and the design of micro/nano fluid devices based on black phosphorus materials.
-
Keywords:
- black phosphorus /
- anisotropy /
- solid-liquid interface /
- molecular dynamics
[1] Keim N C, Arratia P E 2014 Phys. Rev. Lett. 112 028302
 Google Scholar
Google Scholar
[2] Naumis G G, Barraza-Lopez S, Oliva-Leyva M, Terrones H 2017 Rep. Prog. Phys. 80 096501
 Google Scholar
Google Scholar
[3] Paul J T, Singh A K, Dong Z, Zhuang H, Revard B C, Rijal B, Ashton M, Linscheid A, Blonsky M, Gluhovic D, Guo J, Hennig R G 2017 J. Phys.: Condens. Matter 29 473001
 Google Scholar
Google Scholar
[4] 胡小唐, 李源, 饶志军, 胡春光, 傅星 2004 纳米技术与精密工程 2 1
Hu X T, Li Y, Rao Z J, Hu C G, Fu X 2004 Nanotechnology and Precision Engineering 2 1
[5] 周兆英, 杨兴 2003 仪表技术与传感器 2 1
 Google Scholar
Google Scholar
Zhou Z Y, Yang X 2003 Instrument Technique and Sensor 2 1
 Google Scholar
Google Scholar
[6] 严宇才, 张端 2011 电子工业专用设备 40 1
 Google Scholar
Google Scholar
Yan Y C, Zhang R 2011 Equipment for Electronic Products Manufacturing 40 1
 Google Scholar
Google Scholar
[7] Wu L, Deng D, Jin J, Lu X B, Chen J P 2012 Biosens. Bioelectron. 35 193
 Google Scholar
Google Scholar
[8] Khan M, Misra S K, Wang Z, Daza E, Schwartz-Duval A, Kus M J, Pan D 2017 Anal. Chem. 89 2107
 Google Scholar
Google Scholar
[9] Narang J, Malhotra N, Singhal C, Mathur A, Chakraborty D, Anil A, Ingle A, Pundir C S 2017 Biosens. Bioelectron. 88 249
 Google Scholar
Google Scholar
[10] Connacher W, Zhang N, An H, Mei J Y, Zhang S, Gopesh T, Friend J 2018 Lab Chip 10 1039
[11] Soong C Y, Yen T H, Tzeng P Y 2007 Phys. Rev. E 76 036303
 Google Scholar
Google Scholar
[12] Sofos F D, Karakasidis T E, Antonios L 2009 Phys. Rev. E 79 026305
 Google Scholar
Google Scholar
[13] Turlo V, Politano O, Baras F 2015 Acta Mater. 99 363
 Google Scholar
Google Scholar
[14] Balasubramanian S, Mundy C J 1999 Bull. Mater. Sci. 22 873
 Google Scholar
Google Scholar
[15] Wang Z, Jia H, Zheng X, Yang R, Wang Z F, Ye G J, Chen X H, Shan J, Feng, P X L 2015 Nanoscale 7 877
 Google Scholar
Google Scholar
[16] Li L, Guo J Y, Tran V, Tran V, Fei R, Zhang Y 2015 Nat. Nanotechnol. 10 608
 Google Scholar
Google Scholar
[17] Wang X M, Jones A M, Seyler K L, Tran V, Jia Y C, Zhao H, Wang H, Yang L, Xu X D, Xia F N 2015 Nat. Nanotechnol. 10 517
 Google Scholar
Google Scholar
[18] 袁振洲, 刘丹敏, 田楠, 张国庆, 张永哲 2016 化学学报 74 488
Yuan Z Z, Liu D M, Tian N, Zhang G Q, Zhang Y Z 2016 Acta Chimica Sinica 74 488
[19] Chen H, Huang P, Guo D, Xie G X 2016 J. Phys. Chem. C 120 29491
 Google Scholar
Google Scholar
[20] Liu H, Neal A T, Zhu Z, Luo Z, Xu X F, Tomanek D, Ye P D 2014 ACS Nano 8 4033
 Google Scholar
Google Scholar
[21] Xia F, Wang H, Jia Y 2014 Nat. Commun. 5 4458
 Google Scholar
Google Scholar
[22] Fernández-Escamilla H N, Quijano-Briones J J, Tlahuice-Flores A 2016 Phys. Chem. Chem. Phys. 18 12414
 Google Scholar
Google Scholar
[23] Cai K, Wan J, Wei N, Qin Q H 2016 Nanotechnology 27 275701
 Google Scholar
Google Scholar
[24] Horn H W, Swope W C, Pitera J W, Madura J D, Dick T J, Hura G L, Head-Gordon T 2004 J. Chem. Phys. 120 9665
 Google Scholar
Google Scholar
[25] Ryckaert J P, Ciccotti G, Berendsen H J C 1977 J. Comput. Phys. 23 327
 Google Scholar
Google Scholar
[26] Cai K, Liu L, Jiao S, Qin Q H 2017 Mater. Des. 121 406
 Google Scholar
Google Scholar
[27] Zhang H W, Ye H F, Zheng Y G, Zhang Z 2011 Microfluid. Nanofluid. 10 403
 Google Scholar
Google Scholar
[28] Thompson P A, Troian S M 1997 Nature 63 360
[29] Cao B Y, Chen M, Guo Z Y 2006 Phys. Rev. E 74 066311
 Google Scholar
Google Scholar
[30] Zhang H W, Zhang Z Q, Zheng Y G, Wang L, Wang J B 2010 Phys. Rev. E 81 066303
 Google Scholar
Google Scholar
[31] Zhang Z Q, Liu H L, Liu Z, Zhang Z, Cheng G G, Wang X D, Ding J N 2019 Appl. Surf. Sci. 475 857
 Google Scholar
Google Scholar
[32] Koplik J, Banavar J R, Willemsen J F 1988 Phys. Rev. Lett. 60 1282
 Google Scholar
Google Scholar
-
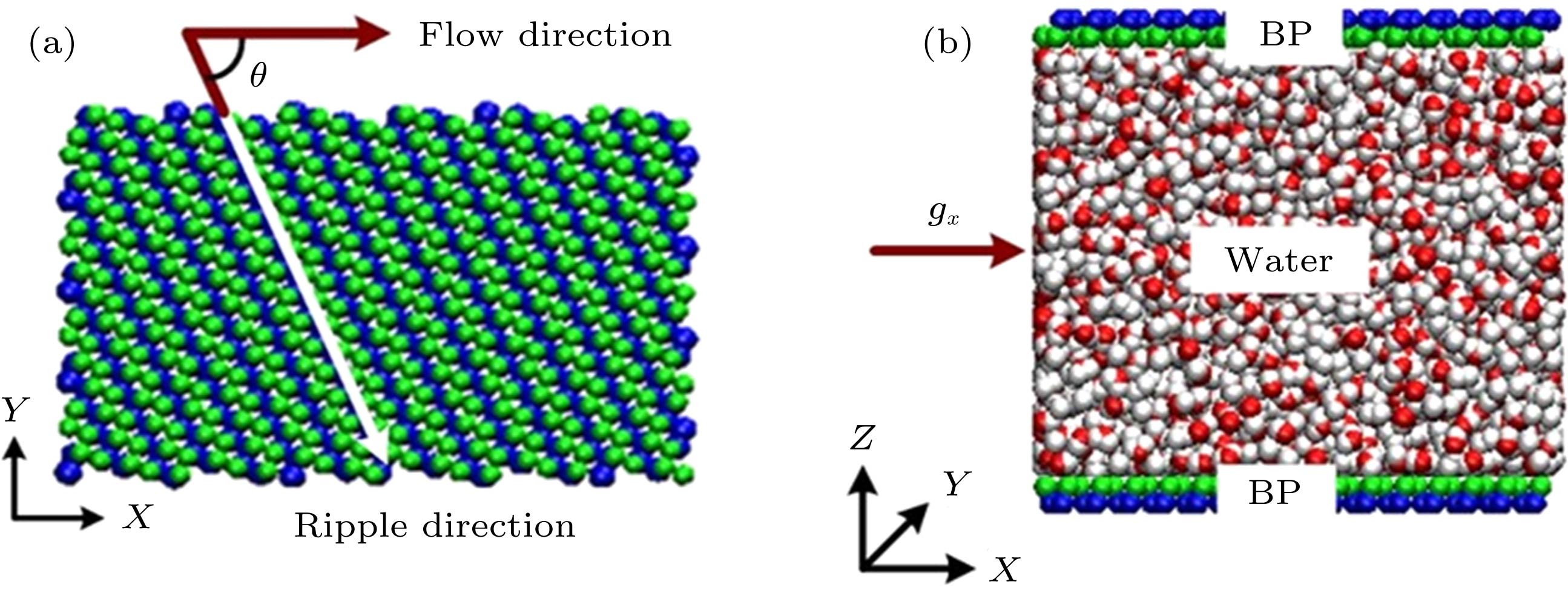
图 1 (a)单层黑磷模型图, 其中手性角度θ指黑磷褶皱方向与水分子流动方向夹角; (b)黑磷纳米通道内水分子流动的Poiseuille流模型图
Fig. 1. (a) Monolayer black phosphorus models, chiral angle θ is the intersection angle between water flow direction adjacent the top plate and the ripple direction of BP monolayer; (b) poiseuille flow model of water molecules in black phosphorus nanochannels.
图 4 速度分布图及势能云图 (a) 模型手性角度为37.4°时水分子的速度分布; (b) 模型手性角度为66.6°时水分子的速度分布; (c) 模型手性角度为90°时水分子的速度分布; (d)模型手性角度为90°时的势能分布云图
Fig. 4. Velocity distribution diagram and potential energy cloud diagram: (a) The velocity distribution of water molecules when the chiral angle of the model is 37.4°; (b) the velocity distribution of water molecules when the chiral angle of the model is 66.6°; (c) the velocity distribution of water molecules when the chiral angle of the model is 90°; (d) potential energy cloud diagram when the chiral angle of the model is 90°.
表 1 L-J势能函数的参数值
Table 1. Parameter values of L-J potential function
Atoms ε/kcal·mol–1 σ/Å P-P 0.36760 3.43800 O-O 0.16275 3.16435 P-O 0.24460 3.30120 表 2 不同手性情况中, 不同加速度对应的水分子边界滑移速度VS统计表
Table 2. Statistical table of water molecule boundary slip velocity VS corresponding to different accelerations in different chiral conditions.
gx/m·s–1 Angle/(°) 0 37.4 66.6 90 1.0 × 1012 6.3305 5.7990 4.7818 3.5462 1.5 × 1012 8.9847 8.7979 6.8867 5.8156 2.0 × 1012 13.4912 12.9694 10.6995 7.5839 表 3 不同手性的模拟系统在不同加速度条件下的水分子黏度系数μ分布表
Table 3. Distribution of water molecular viscosity coefficient μ of simulation systems with different chirality under different acceleration conditions.
gx/m·s–1 Angle/(°) 0 37.4 66.6 90 1.0 × 1012 0.1182 0.1209 0.1116 0.1212 1.5 × 1012 0.1193 0.1173 0.1123 0.1168 2.0 × 1012 0.1171 0.1203 0.1201 0.1183 表 4 不同纳米通道宽度内水分子的边界滑移表
Table 4. Boundary slip of water molecules at different nanochannels widths.
H/nm 3 4 5 6 VS/m·s–1 4.0267 4.3547 5.8005 7.5839 表 5 不同黑磷层数纳米通道模型中流固界面参数对比
Table 5. Comparison of the interfacial parameters for the models with different BP layers.
VS/m·s–1 μ/mPa·s Ew-BP/kcal·mol–1·nm–2 Monolayer Bilayer Monolayer Bilayer Monolayer Bilayer 0° 13.4912 12.9256 0.1171 0.1216 –13.7663 –13.9138 37.4° 12.9694 12.4460 0.1203 0.1204 –13.7797 –13.9285 -
[1] Keim N C, Arratia P E 2014 Phys. Rev. Lett. 112 028302
 Google Scholar
Google Scholar
[2] Naumis G G, Barraza-Lopez S, Oliva-Leyva M, Terrones H 2017 Rep. Prog. Phys. 80 096501
 Google Scholar
Google Scholar
[3] Paul J T, Singh A K, Dong Z, Zhuang H, Revard B C, Rijal B, Ashton M, Linscheid A, Blonsky M, Gluhovic D, Guo J, Hennig R G 2017 J. Phys.: Condens. Matter 29 473001
 Google Scholar
Google Scholar
[4] 胡小唐, 李源, 饶志军, 胡春光, 傅星 2004 纳米技术与精密工程 2 1
Hu X T, Li Y, Rao Z J, Hu C G, Fu X 2004 Nanotechnology and Precision Engineering 2 1
[5] 周兆英, 杨兴 2003 仪表技术与传感器 2 1
 Google Scholar
Google Scholar
Zhou Z Y, Yang X 2003 Instrument Technique and Sensor 2 1
 Google Scholar
Google Scholar
[6] 严宇才, 张端 2011 电子工业专用设备 40 1
 Google Scholar
Google Scholar
Yan Y C, Zhang R 2011 Equipment for Electronic Products Manufacturing 40 1
 Google Scholar
Google Scholar
[7] Wu L, Deng D, Jin J, Lu X B, Chen J P 2012 Biosens. Bioelectron. 35 193
 Google Scholar
Google Scholar
[8] Khan M, Misra S K, Wang Z, Daza E, Schwartz-Duval A, Kus M J, Pan D 2017 Anal. Chem. 89 2107
 Google Scholar
Google Scholar
[9] Narang J, Malhotra N, Singhal C, Mathur A, Chakraborty D, Anil A, Ingle A, Pundir C S 2017 Biosens. Bioelectron. 88 249
 Google Scholar
Google Scholar
[10] Connacher W, Zhang N, An H, Mei J Y, Zhang S, Gopesh T, Friend J 2018 Lab Chip 10 1039
[11] Soong C Y, Yen T H, Tzeng P Y 2007 Phys. Rev. E 76 036303
 Google Scholar
Google Scholar
[12] Sofos F D, Karakasidis T E, Antonios L 2009 Phys. Rev. E 79 026305
 Google Scholar
Google Scholar
[13] Turlo V, Politano O, Baras F 2015 Acta Mater. 99 363
 Google Scholar
Google Scholar
[14] Balasubramanian S, Mundy C J 1999 Bull. Mater. Sci. 22 873
 Google Scholar
Google Scholar
[15] Wang Z, Jia H, Zheng X, Yang R, Wang Z F, Ye G J, Chen X H, Shan J, Feng, P X L 2015 Nanoscale 7 877
 Google Scholar
Google Scholar
[16] Li L, Guo J Y, Tran V, Tran V, Fei R, Zhang Y 2015 Nat. Nanotechnol. 10 608
 Google Scholar
Google Scholar
[17] Wang X M, Jones A M, Seyler K L, Tran V, Jia Y C, Zhao H, Wang H, Yang L, Xu X D, Xia F N 2015 Nat. Nanotechnol. 10 517
 Google Scholar
Google Scholar
[18] 袁振洲, 刘丹敏, 田楠, 张国庆, 张永哲 2016 化学学报 74 488
Yuan Z Z, Liu D M, Tian N, Zhang G Q, Zhang Y Z 2016 Acta Chimica Sinica 74 488
[19] Chen H, Huang P, Guo D, Xie G X 2016 J. Phys. Chem. C 120 29491
 Google Scholar
Google Scholar
[20] Liu H, Neal A T, Zhu Z, Luo Z, Xu X F, Tomanek D, Ye P D 2014 ACS Nano 8 4033
 Google Scholar
Google Scholar
[21] Xia F, Wang H, Jia Y 2014 Nat. Commun. 5 4458
 Google Scholar
Google Scholar
[22] Fernández-Escamilla H N, Quijano-Briones J J, Tlahuice-Flores A 2016 Phys. Chem. Chem. Phys. 18 12414
 Google Scholar
Google Scholar
[23] Cai K, Wan J, Wei N, Qin Q H 2016 Nanotechnology 27 275701
 Google Scholar
Google Scholar
[24] Horn H W, Swope W C, Pitera J W, Madura J D, Dick T J, Hura G L, Head-Gordon T 2004 J. Chem. Phys. 120 9665
 Google Scholar
Google Scholar
[25] Ryckaert J P, Ciccotti G, Berendsen H J C 1977 J. Comput. Phys. 23 327
 Google Scholar
Google Scholar
[26] Cai K, Liu L, Jiao S, Qin Q H 2017 Mater. Des. 121 406
 Google Scholar
Google Scholar
[27] Zhang H W, Ye H F, Zheng Y G, Zhang Z 2011 Microfluid. Nanofluid. 10 403
 Google Scholar
Google Scholar
[28] Thompson P A, Troian S M 1997 Nature 63 360
[29] Cao B Y, Chen M, Guo Z Y 2006 Phys. Rev. E 74 066311
 Google Scholar
Google Scholar
[30] Zhang H W, Zhang Z Q, Zheng Y G, Wang L, Wang J B 2010 Phys. Rev. E 81 066303
 Google Scholar
Google Scholar
[31] Zhang Z Q, Liu H L, Liu Z, Zhang Z, Cheng G G, Wang X D, Ding J N 2019 Appl. Surf. Sci. 475 857
 Google Scholar
Google Scholar
[32] Koplik J, Banavar J R, Willemsen J F 1988 Phys. Rev. Lett. 60 1282
 Google Scholar
Google Scholar
计量
- 文章访问数: 21295
- PDF下载量: 91
- 被引次数: 0













 下载:
下载: