-
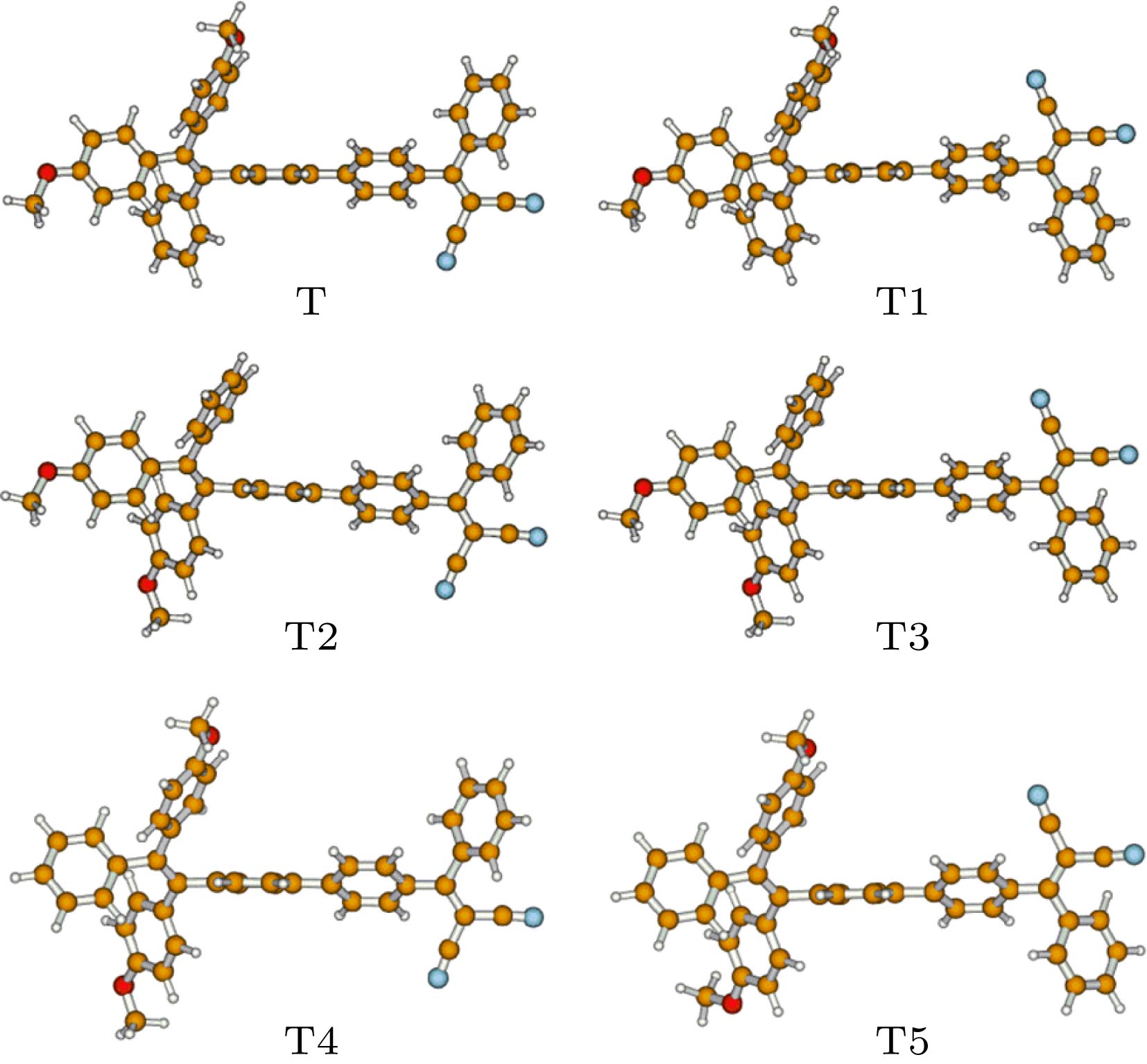
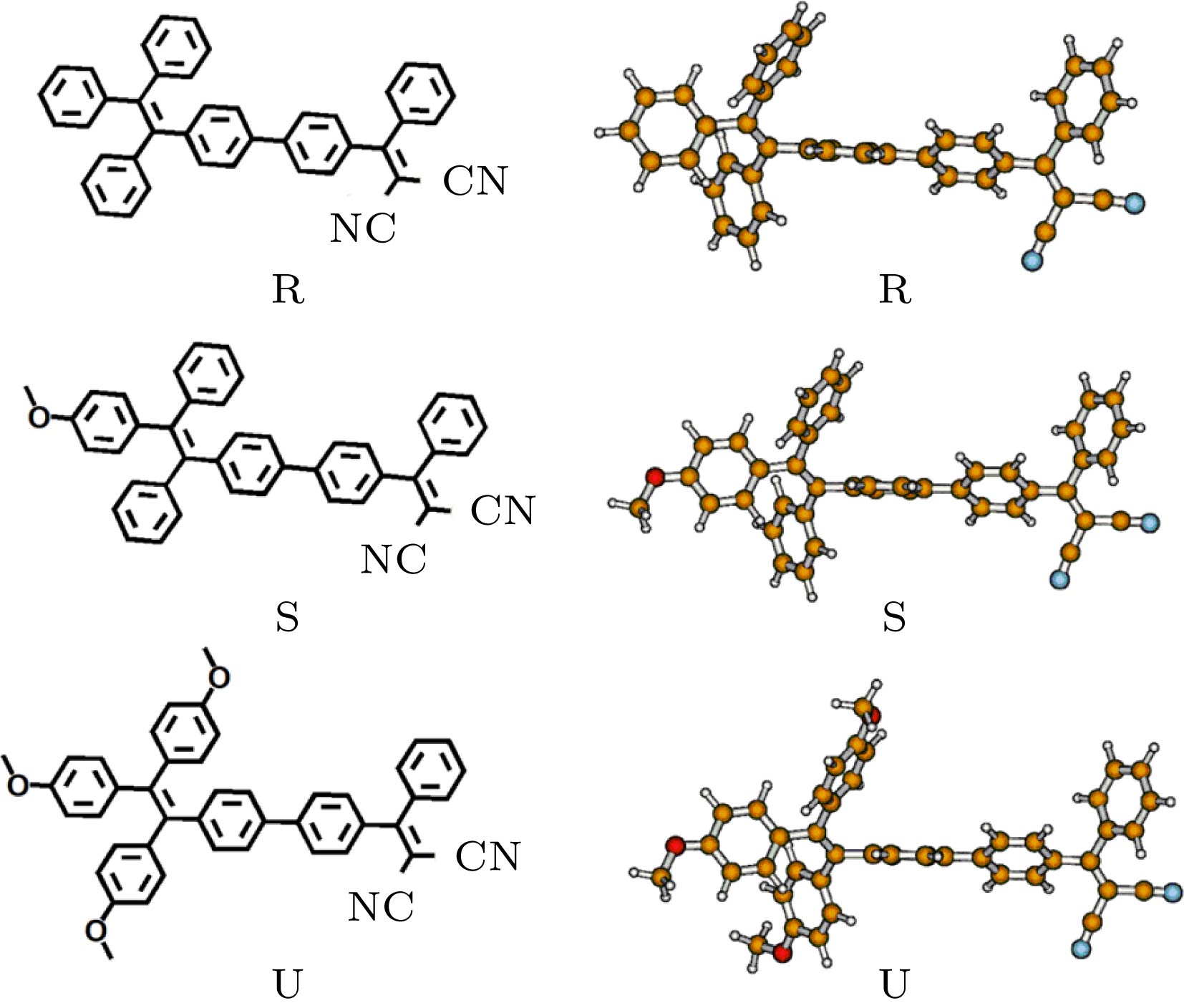
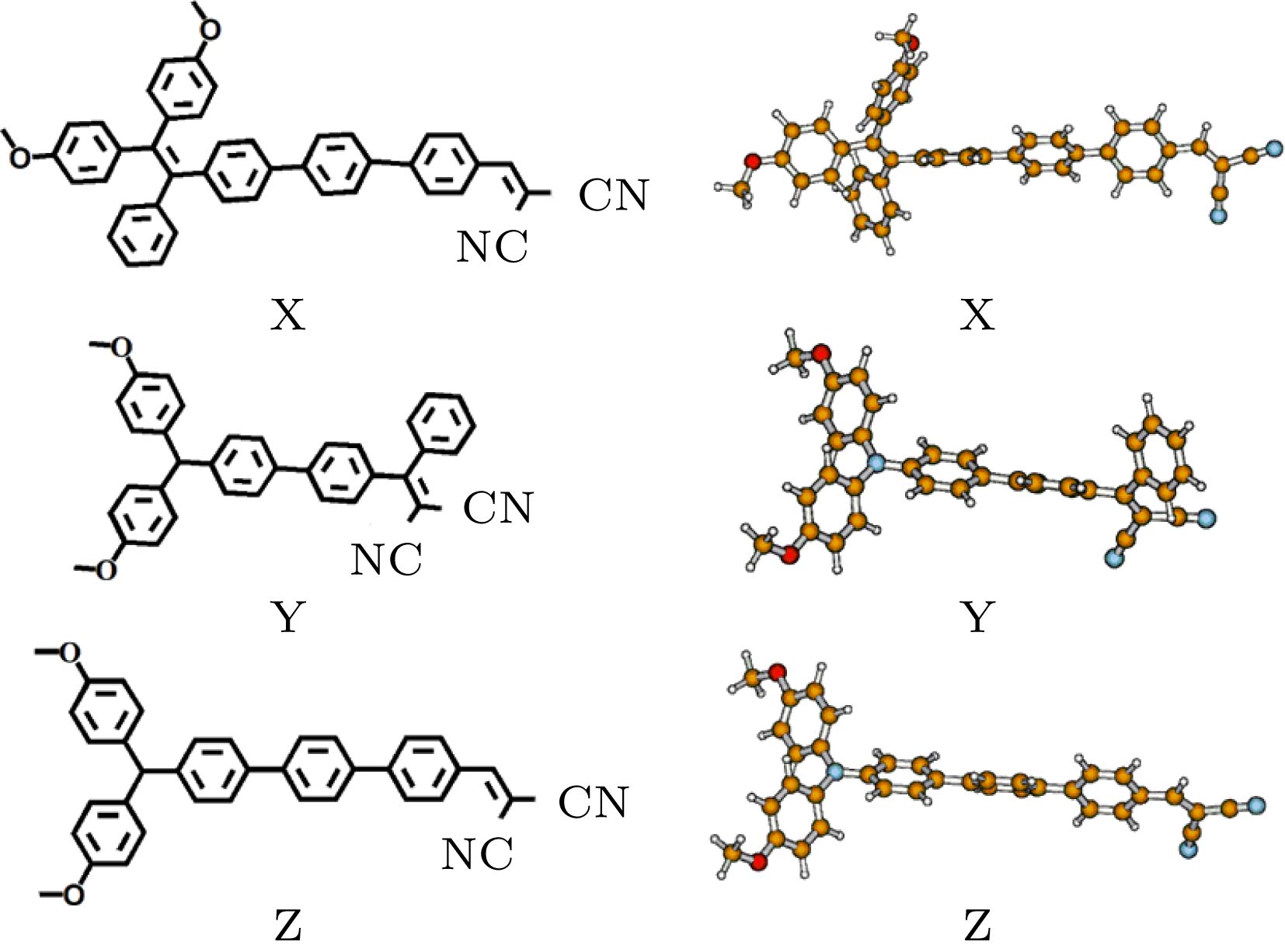
在杂化密度泛函水平上, 利用响应函数方法, 计算了一类四苯基乙烯衍生物的双光子吸收性质. 考虑了四苯基乙烯上给电子基团的位置和数目对双光子吸收性质的影响. 并且, 根据实验者采用的分子, 通过增加分子的平面性和共轭长度, 以及增强给体强度, 理论设计了三种分子结构, 并计算了它们的双光子吸收性质. 结果表明, 给体位置和数目对双光子吸收性质有重要影响. 位于分子末端的给体取代基能有效提高双光子吸收强度. 随着给体数目的增加, 双光子吸收波长发生红移. 在四苯基乙烯的不同侧位上添加给体取代基对双光子吸收性质的影响有明显差异. 与实验者采用的分子相比, 理论设计的分子结构双光子吸收截面均明显增大. 当三苯胺基代替四苯基乙烯基之后, 双光子吸收峰发生较大红移, 双光子吸收截面明显增大.Organic materials with strong two-photon absorption response and aggregation induced emission have aroused a great deal of interest in recent years, for their many potential applications such as two-photon fluorescence microscopy, up-conversion laser, photodynamic therapy, etc. The tetraphenylethylene units are usually employed in two-photon absorption and aggregation induced emission materials because of their good electron-donating capability and special propeller starburst structures. Theoretical study on the relationship between molecular structure and two-photon absorption property is of great importance for guiding the experimental design and synthesis of functional materials. In this paper, the two-photon absorption properties of a series of organic molecules containing tetraphenylethylene and cyano groups are studied by employing the density functional response theory in combination with the polarizable continuum model. The molecular geometries are optimized at a hybrid B3LYP level with 6-31g(d, p) basis set in the Gaussian 16 program. The two-photon absorption cross sections are calculated by response theory through using the CAM-B3LYP functional with 6-31g(d) basis set in the Dalton program. The effect of donor position and number on two-photon absorption properties are investigated. In addition, by increasing the planarity and conjugated length of the molecule, as well as by enhancing the strength of the electron donor, we design three molecular structures and calculate their two-photon absorption properties. The results show that the donor position and number have important effects on two-photon absorption properties. The methoxy donor at the end of the molecule can increase the two-photon absorption intensity effectively. As the number of substituents increases, the position of the two-photon absorption peak is red-shifted. The effects of adding electron donor groups on different side positions have a significant difference in the two-photon absorption property. Comparing with the experimental molecules, the two-photon absorption cross sections of the designed molecules are greatly enhanced. When the tetraphenylethylene group is replaced by the triphenylamine group, the two-photon absorption peak is greatly red-shifted, and the two-photon absorption intensity is significantly increased. Since all of these molecules contain tetraphenylethylene or triphenylamine group with propeller structure, they can have both two-photon absorption and aggregation induced emission properties. This study provides theoretical guidelines for synthesizing a new type of active two-photon absorption and aggregation induced emission material.
-
Keywords:
- tetraphenylethylene /
- two-photon absorption /
- donor position /
- donor number
[1] Kim S, Zheng Q, He G S, Bharali D J, Pudavar H E, Baev A, Prasad P N 2006 Adv. Funct. Mater. 16 2317
[2] Kim S, Pudavar H E, Bonoiu A, Prasad P N 2007 Adv. Mater. 19 3791
[3] Hu R, Maldonado J, Rodriguez M, Deng C, Jim C W, Lam J Y, Yuen M F, Ramos-Ortiz G, Tang B Z 2012 J. Mater. Chem. 22 232
 Google Scholar
Google Scholar
[4] Zhang Y, Li J, Tang B Z, Wong K S 2014 J. Phys. Chem. C 118 26981
[5] Jiang Y, Wang Y, Hua J, Tang J, Li B, Qian S, Tian H 2010 Chem. Commun. 46 4689
 Google Scholar
Google Scholar
[6] Wang B, Wang Y, Hua J, Jiang Y, Huang J, Qian S, Tian H 2011 Chem. Eur. J. 17 2647
 Google Scholar
Google Scholar
[7] Xu B, Xie M, He J, Xu B, Chi Z, Tian W, Jiang L, Zhao F, Liu S, Zhang Y 2013 Chem. Commun. 49 273
 Google Scholar
Google Scholar
[8] Jiang T, Qu Y, Li B, Gao Y, Hua J 2015 RSC Adv. 5 1500
[9] Qu C, Gao Z, Chen Y 2018 J. Lumin. 194 40
 Google Scholar
Google Scholar
[10] Gu B, Wu W, Xu G, Feng G, Yin F, Chong P H J, Qu J, Yong K T, Liu B 2017 Adv. Mater. 29 1701076
 Google Scholar
Google Scholar
[11] He G S, Tan L S, Zheng Q, Prasad P N 2008 Chem. Rev. 108 1245
 Google Scholar
Google Scholar
[12] Pawlicki M, Collins H A, Denning R G, Anderson H L 2009 Angew. Chem. Int. Ed. 48 3244
 Google Scholar
Google Scholar
[13] Hong Y, Lam J W Y, Tang B Z 2009 Chem. Commun. 29 4332
[14] Hong Y, Lam J W Y, Tang B Z 2011 Chem. Soc. Rev. 40 5361
 Google Scholar
Google Scholar
[15] Wang F Q, Zhao K, Zhu M Y, Wang C K 2016 J. Phys. Chem. B 120 9708
 Google Scholar
Google Scholar
[16] Song J, Zhao K, Zhang H, Wang C K 2019 Mol. Phys. 117 672
 Google Scholar
Google Scholar
[17] Katan C, Terenziani F, Mongin O, Werts M H V, Porrès L,Pons T, Mertz J, Tretiak S, Blancharddesce M 2005 J. Phys. Chem. A 109 3024
 Google Scholar
Google Scholar
[18] Terenziani F, Morone M, Gmouh S, Blancharddesce M 2006 Chem. Phys. Chem. 7 685
 Google Scholar
Google Scholar
[19] Chattopadhyaya M, Alam M M, Chakrabarti S 2011 J. Phys. Chem. A 115 2607
 Google Scholar
Google Scholar
[20] Luo Y, Norman P, Macak P, Ågren H 2000 J. Phys. Chem. A 104 4718
 Google Scholar
Google Scholar
[21] Olsen J, Jørgensen P 1985 J. Chem. Phys. 82 3235
 Google Scholar
Google Scholar
[22] Monson P R, Mcclain W M 1970 J. Chem. Phys. 53 29
 Google Scholar
Google Scholar
[23] Gaussian 16, Revision A.03, Gaussian, Inc., Wallingford CT, 2016 http://www.gaussian.com/ [2019-3-31]
[24] Dalton, a Molecular Electronic Structure Program, Release DALTON2013.0, 2013 http://daltonprogram.org/ [2019-3-31]
[25] Zhao K, Liu P W, Wang C K, Luo Y 2010 J. Phys. Chem. B 114 10814
 Google Scholar
Google Scholar
[26] 武香莲, 赵珂, 贾海洪, 王富青 2015 64 233301
 Google Scholar
Google Scholar
Wu X L, Zhao K, Jia H H, Wang F Q 2015 Acta Phys. Sin. 64 233301
 Google Scholar
Google Scholar
[27] Zhu M Y, Zhao K, Song J, Wang C K 2018 Chin. Phys. B 27 023302
 Google Scholar
Google Scholar
[28] Zhao K, Song J, Zhu M Y, Zhang H, Wang C K 2018 Chin. Phys. B 27 103301
 Google Scholar
Google Scholar
[29] Zhang Y J, Zhang Q Y, Ding H J, Song X N, Wang C K 2015 Chin. Phys. B 24 023301
 Google Scholar
Google Scholar
[30] Chung S J, Kim K S, Lin T C, He G S, Swiatkiewicz J, Prasad P N 1999 J. Phys. Chem. B. 103 10741
 Google Scholar
Google Scholar
[31] Adronov A, Fréchet J M J, He G S, Kim K S, Chung S J, Swiatkiewicz J, Prasad P N 2000 Chem. Mater. 12 2838
 Google Scholar
Google Scholar
[32] Chung S J, Lin T C, Kim K S, He G S, Swiatkiewicz J, Prasad P N, Baker G A, Bright F V 2001 Chem. Mater. 13 4071
 Google Scholar
Google Scholar
-
表 1 分子六个最低激发态的TPA波长
${\lambda _{{\rm{tp}}}}$ (nm)和TPA截面$\sigma $ (GM)Table 1. The TPA wavelength
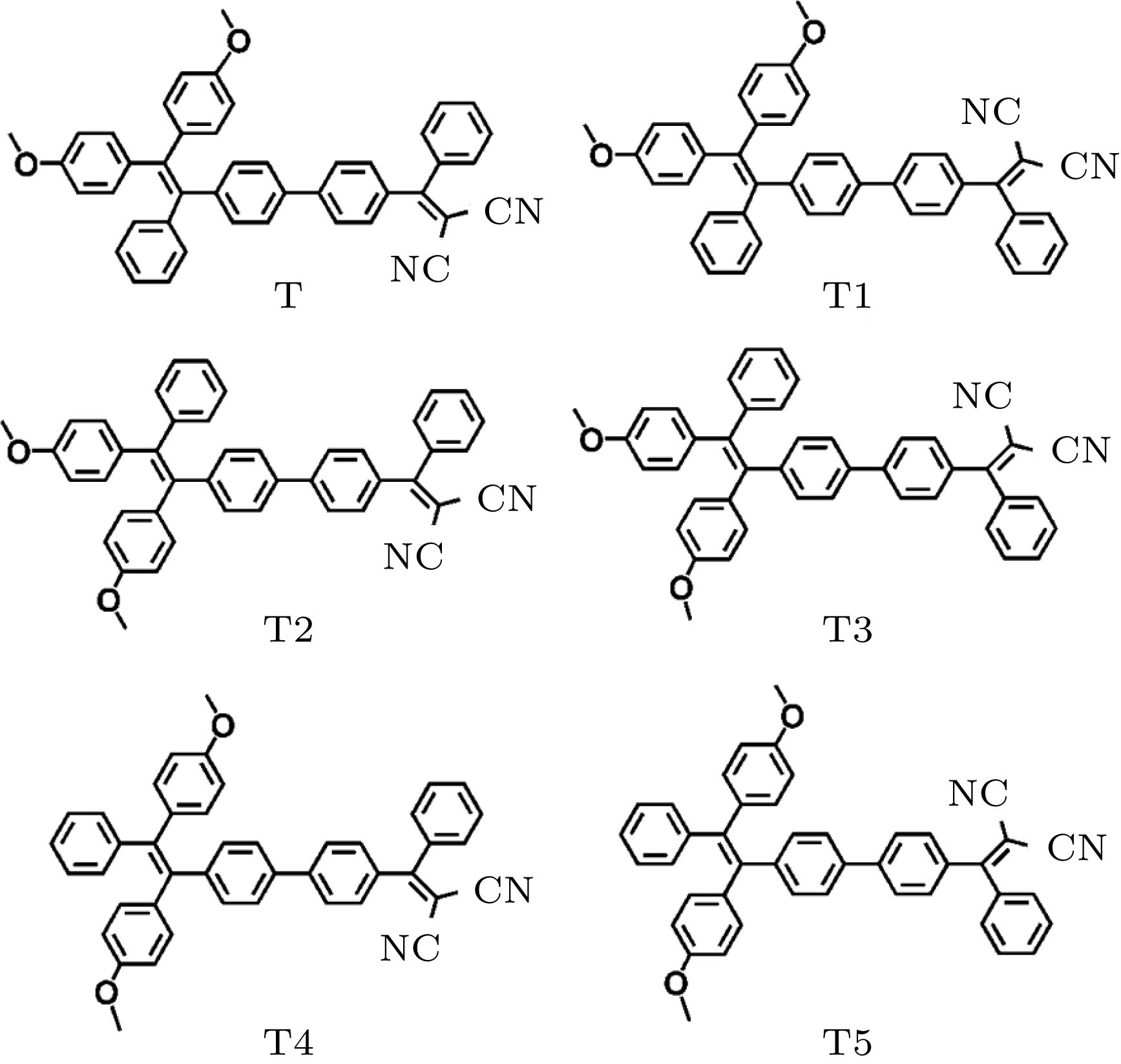
${\lambda _{{\rm{tp}}}}$ (nm) and the TPA cross section$\sigma $ (GM) of the lowest six excited states.T T1 T2 T3 T4 T5 ${\lambda _{{\rm{tp}}}}$/nm $\sigma $/GM ${\lambda _{{\rm{tp}}}}$/nm $\sigma $/GM ${\lambda _{{\rm{tp}}}}$/nm $\sigma $/GM ${\lambda _{{\rm{tp}}}}$/nm $\sigma $/GM ${\lambda _{{\rm{tp}}}}$/nm $\sigma $/GM ${\lambda _{{\rm{tp}}}}$/nm $\sigma $/GM S1 743 719 743 696 740 707 738 672 743 638 743 629 S2 650 4 649 2 651 10 651 6 652 8 653 4 S3 612 944 612 858 614 893 612 873 615 880 615 827 S4 572 305 572 334 578 212 575 259 578 136 580 175 S5 556 3 556 5 557 4 556 5 557 4 557 5 S6 540 15 540 9 542 2 540 5 542 3 541 2 表 2 分子六个最低激发态的TPA波长
${\lambda _{{\rm{tp}}}}$ (nm)和TPA截面$\sigma $ (GM)Table 2. The TPA wavelength
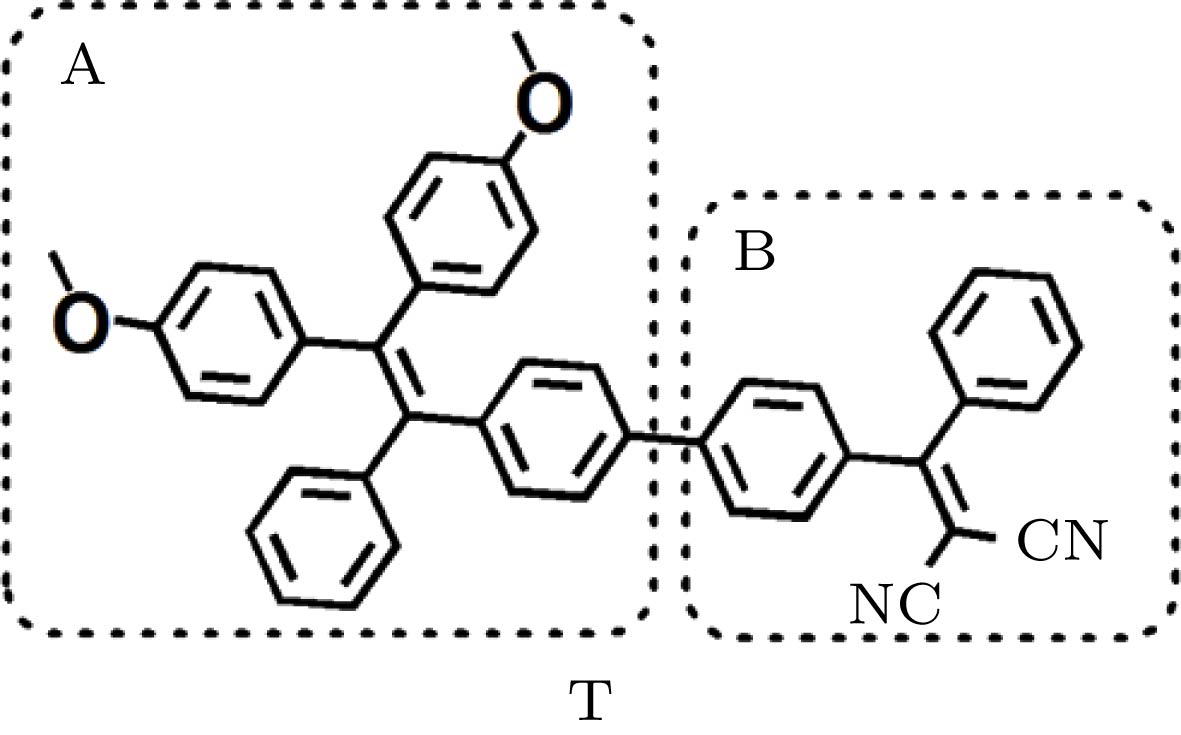
${\lambda _{{\rm{tp}}}}$ (nm) and the TPA cross section$\sigma $ (GM) of the lowest six excited states.R S U ${\lambda _{{\rm{tp}}}}$/nm $\sigma $/GM ${\lambda _{{\rm{tp}}}}$/nm $\sigma $/GM ${\lambda _{{\rm{tp}}}}$/nm $\sigma $/GM S1 719 500 729 646 754 769 S2 631 27 642 19 658 7 S3 595 597 603 785 621 931 S4 557 4 567 640 580 118 S5 556 853 557 4 557 4 S6 540 99 541 29 541 11 表 3 分子各部分的基态电荷和第一激发态电荷(单位: e)
Table 3. Net charges (unit: e) for divided parts of the molecules in the ground states and in the first excited states.
QA0/e QA1/e QA/e QB0/e QB1/e QB/e T 0.0298 0.3978 0.3680 – 0.0298 – 0.3978 – 0.3680 T2 0.0292 0.3901 0.3609 – 0.0292 – 0.3901 – 0.3609 T4 0.0294 0.4066 0.3772 – 0.0294 – 0.4066 – 0.3772 R 0.0271 0.3136 0.2865 – 0.0271 – 0.3136 – 0.2865 S 0.0284 0.3526 0.3242 – 0.0284 – 0.3526 – 0.3242 U 0.0305 0.4340 0.4305 – 0.0305 – 0.4340 – 0.4305 表 4 分子六个最低激发态的TPA波长
${\lambda _{{\rm{tp}}}}$ (nm)和TPA截面$\sigma $ (GM)Table 4. The TPA wavelength
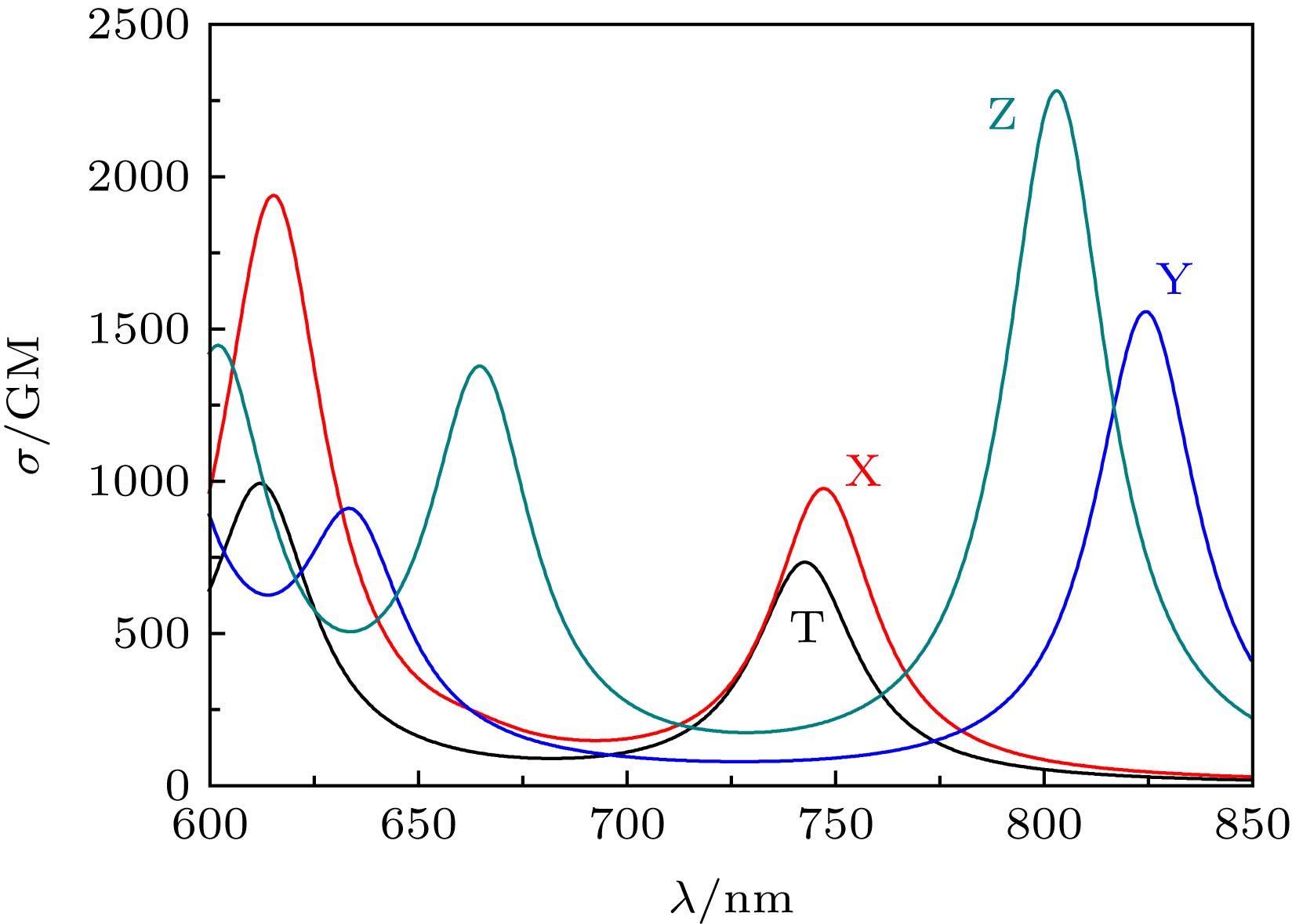
${\lambda _{{\rm{tp}}}}$ (nm) and the TPA cross section$\sigma $ (GM) of the lowest six excited states.X Y Z ${\lambda _{{\rm{tp}}}}$/nm $\sigma $/GM ${\lambda _{{\rm{tp}}}}$/nm $\sigma $/GM ${\lambda _{{\rm{tp}}}}$/nm $\sigma $/GM S1 747 947 824 1543 802 2259 S2 663 40 634 737 602 1362 S3 615 1906 579 1763 582 1 S4 560 1 576 91 540 57 S5 542 65 570 606 527 738 S6 525 502 560 50 546 4 -
[1] Kim S, Zheng Q, He G S, Bharali D J, Pudavar H E, Baev A, Prasad P N 2006 Adv. Funct. Mater. 16 2317
[2] Kim S, Pudavar H E, Bonoiu A, Prasad P N 2007 Adv. Mater. 19 3791
[3] Hu R, Maldonado J, Rodriguez M, Deng C, Jim C W, Lam J Y, Yuen M F, Ramos-Ortiz G, Tang B Z 2012 J. Mater. Chem. 22 232
 Google Scholar
Google Scholar
[4] Zhang Y, Li J, Tang B Z, Wong K S 2014 J. Phys. Chem. C 118 26981
[5] Jiang Y, Wang Y, Hua J, Tang J, Li B, Qian S, Tian H 2010 Chem. Commun. 46 4689
 Google Scholar
Google Scholar
[6] Wang B, Wang Y, Hua J, Jiang Y, Huang J, Qian S, Tian H 2011 Chem. Eur. J. 17 2647
 Google Scholar
Google Scholar
[7] Xu B, Xie M, He J, Xu B, Chi Z, Tian W, Jiang L, Zhao F, Liu S, Zhang Y 2013 Chem. Commun. 49 273
 Google Scholar
Google Scholar
[8] Jiang T, Qu Y, Li B, Gao Y, Hua J 2015 RSC Adv. 5 1500
[9] Qu C, Gao Z, Chen Y 2018 J. Lumin. 194 40
 Google Scholar
Google Scholar
[10] Gu B, Wu W, Xu G, Feng G, Yin F, Chong P H J, Qu J, Yong K T, Liu B 2017 Adv. Mater. 29 1701076
 Google Scholar
Google Scholar
[11] He G S, Tan L S, Zheng Q, Prasad P N 2008 Chem. Rev. 108 1245
 Google Scholar
Google Scholar
[12] Pawlicki M, Collins H A, Denning R G, Anderson H L 2009 Angew. Chem. Int. Ed. 48 3244
 Google Scholar
Google Scholar
[13] Hong Y, Lam J W Y, Tang B Z 2009 Chem. Commun. 29 4332
[14] Hong Y, Lam J W Y, Tang B Z 2011 Chem. Soc. Rev. 40 5361
 Google Scholar
Google Scholar
[15] Wang F Q, Zhao K, Zhu M Y, Wang C K 2016 J. Phys. Chem. B 120 9708
 Google Scholar
Google Scholar
[16] Song J, Zhao K, Zhang H, Wang C K 2019 Mol. Phys. 117 672
 Google Scholar
Google Scholar
[17] Katan C, Terenziani F, Mongin O, Werts M H V, Porrès L,Pons T, Mertz J, Tretiak S, Blancharddesce M 2005 J. Phys. Chem. A 109 3024
 Google Scholar
Google Scholar
[18] Terenziani F, Morone M, Gmouh S, Blancharddesce M 2006 Chem. Phys. Chem. 7 685
 Google Scholar
Google Scholar
[19] Chattopadhyaya M, Alam M M, Chakrabarti S 2011 J. Phys. Chem. A 115 2607
 Google Scholar
Google Scholar
[20] Luo Y, Norman P, Macak P, Ågren H 2000 J. Phys. Chem. A 104 4718
 Google Scholar
Google Scholar
[21] Olsen J, Jørgensen P 1985 J. Chem. Phys. 82 3235
 Google Scholar
Google Scholar
[22] Monson P R, Mcclain W M 1970 J. Chem. Phys. 53 29
 Google Scholar
Google Scholar
[23] Gaussian 16, Revision A.03, Gaussian, Inc., Wallingford CT, 2016 http://www.gaussian.com/ [2019-3-31]
[24] Dalton, a Molecular Electronic Structure Program, Release DALTON2013.0, 2013 http://daltonprogram.org/ [2019-3-31]
[25] Zhao K, Liu P W, Wang C K, Luo Y 2010 J. Phys. Chem. B 114 10814
 Google Scholar
Google Scholar
[26] 武香莲, 赵珂, 贾海洪, 王富青 2015 64 233301
 Google Scholar
Google Scholar
Wu X L, Zhao K, Jia H H, Wang F Q 2015 Acta Phys. Sin. 64 233301
 Google Scholar
Google Scholar
[27] Zhu M Y, Zhao K, Song J, Wang C K 2018 Chin. Phys. B 27 023302
 Google Scholar
Google Scholar
[28] Zhao K, Song J, Zhu M Y, Zhang H, Wang C K 2018 Chin. Phys. B 27 103301
 Google Scholar
Google Scholar
[29] Zhang Y J, Zhang Q Y, Ding H J, Song X N, Wang C K 2015 Chin. Phys. B 24 023301
 Google Scholar
Google Scholar
[30] Chung S J, Kim K S, Lin T C, He G S, Swiatkiewicz J, Prasad P N 1999 J. Phys. Chem. B. 103 10741
 Google Scholar
Google Scholar
[31] Adronov A, Fréchet J M J, He G S, Kim K S, Chung S J, Swiatkiewicz J, Prasad P N 2000 Chem. Mater. 12 2838
 Google Scholar
Google Scholar
[32] Chung S J, Lin T C, Kim K S, He G S, Swiatkiewicz J, Prasad P N, Baker G A, Bright F V 2001 Chem. Mater. 13 4071
 Google Scholar
Google Scholar
计量
- 文章访问数: 13142
- PDF下载量: 89
- 被引次数: 0













 下载:
下载: